Figure 1. Domain organization of FAS α- and β-subunits.
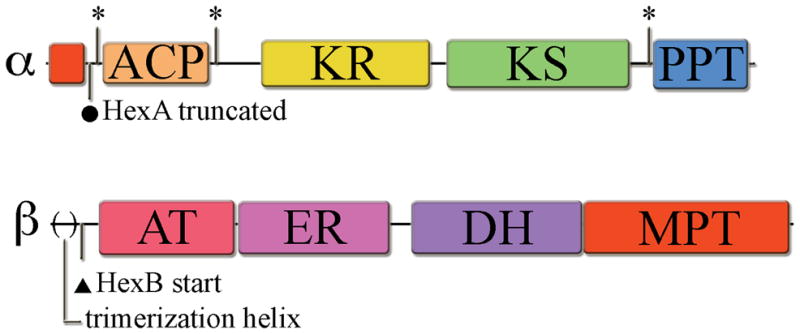
Domains include: acyl-carrier protein (ACP), ketoreductase (KR), ketosynthase (KS), phosphopantetheinyltransferase (PPT), acetyl transacylase (AT), enoyl reductase (ER), dehydrase (DH), and malonyl-palmitoyl transacylase (MPT). (*) indicates cloning sites used within the flexible linker regions surrounding the catalytic domains. (●)indicates the old predicted start site for hexA, which resulted in a truncated gene product. (▲) indicates the predicted hexB start site downstream of the N-terminal structural helix involved in β-subunit trimerization in the primary metabolism FAS. The Aspergillus parasiticus HexA (revised) and HexB proteins (GenBank accession # AY371490) share significant sequence homology to the recently determined crystal structure of similar subunits from Thermomyces lanuginosus (α-subunit, 2UV9_A, 39.4/56.7/11.5 % id/sim/gap, global alignment, http://www.ebi.ac.uk/emboss/align/, and β-subunit, 2UVA_G, 38.1/54.2/11.5, respectively). For a detailed view of domain organization including sequence expansion segments see the T. lanuginosus crystal structure reference [7].
