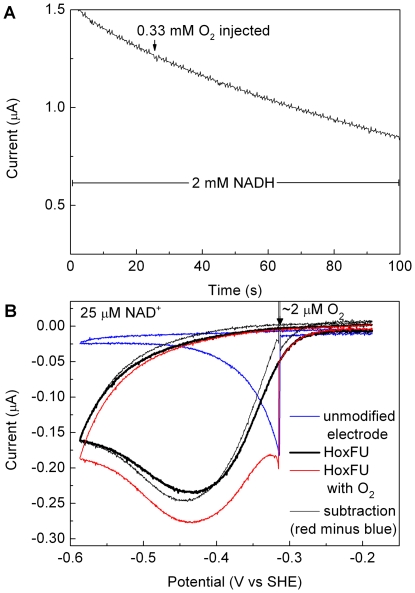
Figure 8. Ability of HoxFU to (A) oxidize NADH and (B) reduce NAD+ in the presence of O2.
In panel A, the potential was held at 243 mV so that the enzyme film oxidises NADH. The drop in current throughout the experiment is attributed to enzyme dissociation from the electrode. An aliquot of air-saturated solution was introduced as indicated. Panel B shows a series of cyclic voltammetric experiments recorded at a scan rate of 1 mV/s with the electrode rotated at 2500 rpm in a solution of 50 mM TrisHCl pH 8.0 containing 25 µM NAD+. The solid black line shows the response for a HoxFU film as the potential is swept from −0.2 V to −0.6 V. The blue line shows the response for an unmodified electrode, as O2 is introduced into the solution during the forward scan as indicated. The red line shows the response when the same amount of O2 is introduced during a potential cycle for an electrode modified with a fresh film of HoxFU (normalized to the current magnitude of the black forward trace at −300 mV). Subtraction of the blue trace (contribution from direct O2 reduction) from the red trace yields the thin black trace which is similar in shape to the solid black trace, confirming that the characteristic shape of the HoxFU electrocatalytic wave is retained in the presence of O2.