Abstract
We examined the ability of the Ebola virus to elicit an antiviral response from plasmacytoid dendritic cells (pDCs). Exposure of pDCs to Ebola virus did not result in significantly higher levels of interferon-α production than the levels in mock-infected cells. After inoculation with Ebola virus under the same conditions, conventional dendritic cells expressed viral proteins whereas pDCs did not, suggesting that the latter cells were not infected. Assessment of the entry of Ebola virus–like particles into pDCs revealed that pDCs are highly impaired for viral entry in comparison with conventional dendritic cells. These observations identify a novel means by which Ebola virus can avoid triggering an antiviral response.
Ebola virus (EBOV) is a lethal pathogen that infects a variety of cell types including macrophages and myeloid dendritic cells [1–3]. The virus can infect these human immune cells without eliciting normal cellular antiviral responses [4, 5], and in vitro studies have implicated the viral proteins VP35 and VP24 as inhibitors of interferon (IFN) production and signaling, respectively (reviewed in [6]). The essential role played by VP35 in pathogenesis is highlighted by the mild disease phenotype and attenuated replication observed in mice and guinea pigs infected with EBOVs encoding mutated VP35s [7, 8].
Although EBOV has been shown to infect conventional dendritic cells (cDCs; a myeloid dendritic cell culture model derived from CD14+ monocytes) without eliciting an antiviral response, similar studies have not been performed with plasmacytoid dendritic cells (pDCs). During infection by negative-strand RNA viruses, cDCs produce IFN following recognition of cytosolic viral RNA by RIG-I, ultimately leading to the activation of TBK1 and IKKε [9], which phosphorylate IRF3. In contrast, pDCs primarily detect viral nucleic acids by endosomal Toll-like receptor 7 (TLR7), which signals through MyD88 to activate either IKKα or IRAK1, which phosphorylate IRF7 [10, 11]. Given that EBOV VP35 has been implicated in inhibiting viral RNA recognition by interfering with RIG-I [12], we asked whether pDCs could produce IFN after infection with EBOV.
METHODS
EBOV Infection of cDCs and pDCs
All experiments involving live EBOV were conducted in the INSERM biosafety level 4 (BSL4) laboratory Jean Merieux in Lyon, France. The cDCs and pDCs were prepared essentially as described elsewhere [13], with the exception that in some instances, peripheral blood mononuclear cells were depleted with anti-CD3, anti-CD8, anti-CD14, anti-CD19, anti-CD56, anti-CD235a, and anti-CD35 beads prior to positive selection of pDCs by use of a BDCA4 cell isolation kit (Miltenyi Biotec). Purified pDCs and cDCs were infected in suspension for 1 hour, centrifuged for 10 minutes at 1000 g, and then resuspended in culture medium RPMI medium with 10% fetal calf serum, plus 10 ng/mL interleukin-3 for pDCs and 10 ng/mL each of granulocyte-macrophage colony-stimulating factor/interleukin-4 for cDCs).
Interferon Enzyme-Linked Immunosorbent Assay
Interferon from the supernatants of infected cells was quantified using an IFN-α enzyme-linked immunosorbent assay (ELISA) kit (Bender MedSystems). The assay was performed within the BSL4 containment facility.
Western Blot
Samples from EBOV-infected cells were incubated in loading buffer (2% sodium dodecyl sulfate [SDS] and 5% β-mercaptoethanol) at 96°C for 15 minutes. Proteins were separated by SDS polyacrylamide gel electrophoresis and subsequently analyzed by Western blot using horse anti-EBOV polyclonal antibody and anti-horse horseradish peroxidase–coupled antibodies (Sigma-Aldrich). The signal was detected using SuperSignal West Dura chemiluminescent substrate (Thermo Scientific).
Viruslike Particle Production
Viruslike particles (VLPs) were produced by transfecting 18 μg of expression plasmids into 107 293T cells in 55-cm2 plates by use of Lipofectamine 2000 (Invitrogen) as recommended by the manufacturer. Twelve micrograms of EBOV VP40 expression plasmid was transfected with 6 μg of EBOV glycoprotein (GP) expression plasmid (producing EBOV GP VLPs), or 6 μg of EBOV VP40 expression plasmid was transfected with 6 μg of influenza A/South Carolina/1/18(H1N1) virus hemagluttinin (HA) and 6 μg of influenza A/South Carolina/1/18(H1N1) virus neuraminidase expression plasmids (producing influenza HA VLPs). Two days after transfection, VLP-containing supernatant was cleared of cellular debris by centrifugation at 200 g. VLPs were isolated by centrifuging the cleared supernatant through a sucrose cushion (20% wt/vol) at 100,000 g for 2 hours at 4°C, washed with ice-cold NTE buffer (10 mmol/L Tris; pH, 7.5; 100 mmol/L sodium chloride; 1 mmol/L ethylenediaminetetraacetic acid), and recovered by centrifugation. VLPs were resuspended in 50–100 μL of NTE buffer and stored at 4°C until used for entry and binding assays.
Entry and Binding Assays
The β-lactamase enzyme was fused to the N-terminus of the Zaire Ebola virus VP40, via the linker peptide GSGGGSGGT. Thus, β-lactamase activity in the cytoplasm of cells (entry of lactamase-VP40) can be measured by fluorescence emission of a membrane-permeable substrate that is retained in the cytoplasm (CCF-2AM; Invitrogen) that normally fluoresces green but fluoresces blue when cleaved by β-lactamase.
For both entry and binding assays, VLP-bound cells were formed by incubating 3 × 106 cells (pDCs or cDCs) with 1 × 107 β-lactamase equivalents of VLPs and spinoculating for 1 hour at 300 g at 10°C. For the entry assay, VLP-bound cells were placed at 37°C in a 5% carbon dioxide incubator for 4 hours to permit entry, and then treated with CCF-2AM substrate (Invitrogen) for 1 hour and analyzed by flow cytometry on a BD LSRII (BD Biosciences). For the binding assay, VLP-bound cells were incubated for 1 hour at 10°C, washed with ice-cold phosphate-buffered saline, and then analyzed for β-lactamase activity by use of a LyticBLAzer BODIPY FL Homogenous assay kit (Invitrogen) according to the manufacturer’s recommended protocol.
RESULTS
Plasmacytoid Dendritic Cells Fail to Produce IFN-α After Exposure to EBOV
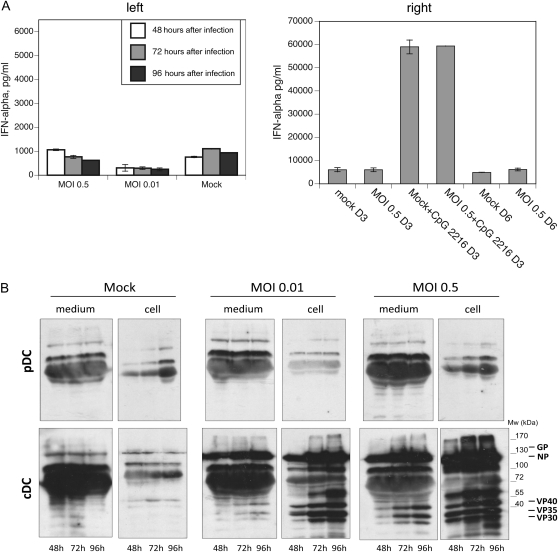
To determine whether pDCs produce IFN in response to EBOV, we harvested cells and supernatants from EBOV-infected human pDCs (multiplicity of infection [MOI], 0.5 or 0.01) 2, 3, and 4 days after infection. ELISA analysis revealed no detectable amounts of IFN-α produced from any of the infected cells relative to mock-infected controls (Figure 1). Incubation of these pDCs with the TLR9 agonist CpG 2216 led to significant IFN-α production, whether or not the pDCs had been previously incubated with EBOV (Figure 1). These results demonstrate that pDCs, which are prolific producers of IFN when they are exposed to RNA viruses, are unable to produce IFN when exposed to EBOV.
Figure 1.
Plasmacytoid dendritic cells (pDCs) do not produce interferon (IFN) upon exposure to Ebola virus and resist infection ex vivo. Supernatants from pDCs exposed to Ebola virus were assayed for IFN-α by enzyme-linked immunosorbent assay (A). For some samples, CpG 2216 was added 3 days after infection (A, right). Lysates were prepared from infected conventional dendritic cells (cDCs) or pDCs at the indicated time points and analyzed for the presence of viral proteins by Western blot analysis (B). The location of viral proteins is indicated. GP, glycoprotein; MOI, multiplicity of infection; Mw, molecular weight; NP, nucleoprotein.
One explanation for the unresponsiveness of pDCs to EBOV is that the virus fails to infect these cells, thereby avoiding the intracellular sensors of viral infection, such as TLRs, RIG-I, or MDA5. Western blot analysis of the infected pDCs or their corresponding supernatants failed to detect the presence of viral proteins in any of the samples (Figure 1). In contrast, cDCs expressed abundant viral antigens for 2–4 days after infection at MOI of 0.01 or 0.5 (Figure 1). The presence of viral proteins in the supernatants of cDCs infected at MOI of 0.5 demonstrates that cDCs are productively infected with EBOV [4, 5, 14]. On the basis of these findings, we conclude that EBOV does not infect pDCs under our experimental conditions.
EBOV-like Particles Fail to Enter pDCs
Next, we asked whether these cells were capable of binding and/or internalizing EBOV-like particles. Viruslike particles produced by transient transfection of 293T cells with the EBOV matrix protein VP40 and the glycoprotein GP (EBOV GP VLPs) have been shown to have similar ultrastructural and immunogenic properties to those of the actual virus. By creating VLPs using a VP40-β lactamase chimera in conjunction with an appropriate fluorogenic substrate, it is possible to study viral entry and binding into permissive cells.
We observe that cDCs readily internalize EBOV GP VLPs, but pDCs do not (Figure 2). To determine whether the pDCs were generally impaired with respect to VLP entry, we prepared an additional set of VLPs containing the HA protein from influenza A virus (HA VLPs). Entry of the HA VLPs was evident in pDCs, demonstrating that these cells were not impaired for viral entry per se.
Figure 2.
EBOV glycoprotein (GP) viruslike particles (VLPs) are readily internalized by conventional dendritic cells (cDCs) but not by plasmacytoid dendritic cells (pDCs). The cDCs and pDCs were incubated with the indicated VLPs (A). The gated region indicates the presence of cytoplasmic β-lactamase substrate (fluorescing at 520 nm) converted to product (fluorescing at 460 nm) in response to internalized VLP Y-Axis indicates fluorescence at 520 nm; X-Axis indicates fluorescence at 460 nm. The cDCs (light gray) and pDCs (dark gray) were incubated with the indicated VLP and lysed in the presence of a fluorogenic β-lactamase substrate (B). HA, hemagluttinin.
Finally, we examined the ability of cDCs and pDCs to bind VLPs, hypothesizing that the impaired entry could be due to weaker binding of EBOV GP VLPs to the different cells. Binding of EBOV GP VLPs to pDCs was low, but it was slightly higher than that to cDCs. In the case of HA VLPs, both cells showed significant binding, with pDCs binding ∼2-fold more HA VLPs than cDCs (Figure 2). Our results suggest that EBOV fails to infect pDCs due to an inability to gain entry into these cells despite the ability of the virus to attach to the cell surface.
DISCUSSION
Entry into a permissive host cell is the first step in the EBOV life cycle, which allows the pathogen access to a biochemical environment that is essential for replication. Many enveloped viruses, such as EBOV, enter using the endocytic machinery of the host cell and subsequently fuse with the endolysosomal membrane, thus escaping into the cytosol, where it can begin replicating its genome [15]. However, this same endocytic process is used by cells of the immune system to sample the extracellular environment for biomolecules indicative of infection, such as lipopolysaccharide, zymosan, and nonself nucleic acids [16]. Positioned within the endosomal system of dendritic cells is a subset of the TLRs (TLR3, TLR7, TLR8, and TLR9), which act as sensors of viral nucleic acids, and the avoidance of these initiators of an antiviral response is necessary if the virus is to successfully infect its host [16].
We observed productive infection of cDCs by EBOV, which correlates with our entry assay using EBOV GP VLPs and cDCs. Conversely, EBOV failed to infect pDCs, which correlates with the inability of EBOV GP VLPs to enter these cells. Recent reports suggest that EBOVs enter macrophages and dendritic cells through macropinocytosis [17, 18]. In comparison with these antigen-presenting cells, pDCs exhibit much lower levels of phagocytosis and endocytosis [19]. If macropinocytosis activity in pDCs is low or absent, then this may explain the poor entry of EBOV GP VLPs into pDCs (Figure 2), as well as the resistance of these cells to EBOV infection (Figure 1). If EBOVs do not enter the endosomal system of pDCs, then viral nucleic acids would not encounter the TLR pathway of these professional IFN-producing cells, thus precluding the initiation of a potential antiviral response.
The failure of EBOV to enter and activate pDCs may effectively delay the host innate immune response such that it is rendered ineffective. It will be of interest to determine whether, during the course of infection in vivo, pDCs can be induced to become permissive for EBOV entry and thereby contribute to the host response to infection. Alternately, it may be possible to inhibit macropinocytosis of macrophages and myeloid dendritic cells to prevent viral entry. In that respect, our VLP entry assay can be used to screen for inhibitors of this pathway that may prove useful as therapeutics.
Funding
This work was supported by the Agence Nationale de la Recherche (grant MIME-006-01 to V. E. V.); Fondation pour la Recherche Médicale en France (grant DMI20091117323 to V. E. V.]; and the National Institutes of Health (grants AI059536 and AI057158 [Northeast Biodefense Center–Lipkin] to C. F. B.).
Acknowledgments
Chantal Rabourdin-Combe, Thomas Duhen, and Sophia Djebali provided helpful discussion and experimental designs. We thank the biosafety team members for their assistance.
References
- 1.Geisbert TW, Hensley LE, Larsen T, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163:2347–70. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takada A, Robison C, Goto H, et al. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci U S A. 1997;94:14764–9. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wool-Lewis RJ, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J Virol. 1998;72:3155–60. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosio CM, Aman MJ, Grogan C, et al. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis. 2003;188:1630–8. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- 5.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol. 2003;170:2797–801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- 6.Basler CF, Amarasinghe GK. Evasion of interferon responses by Ebola and Marburg viruses. J Interferon Cytokine Res. 2009;29:511–20. doi: 10.1089/jir.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartman AL, Bird BH, Towner JS, Antoniadou ZA, Zaki SR, Nichol ST. Inhibition of IRF-3 activation by VP35 is critical for the high level of virulence of ebola virus. J Virol. 2008;82:2699–704. doi: 10.1128/JVI.02344-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prins KC, Delpeut S, Leung DW, et al. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J Virol. 2009;84:3004–15. doi: 10.1128/JVI.02459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoneyama M, Kikuchi M, Natsukawa T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–7. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 10.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–73. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung DW, Prins KC, Borek DM, et al. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat Struct Mol Biol. 2010;17:165–72. doi: 10.1038/nsmb.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A. Interferon-beta pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 2008;4:e1000193. doi: 10.1371/journal.ppat.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta M, Mahanty S, Ahmed R, Rollin PE. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology. 2001;284:20–5. doi: 10.1006/viro.2001.0836. [DOI] [PubMed] [Google Scholar]
- 15.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–33. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 16.Blasius AL, Beutler B. Intracellular Toll-like receptors. Immunity. 2010;32:305–15. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Nanbo A, Imai M, Watanabe S, et al. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010;6:e1001121. doi: 10.1371/journal.ppat.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010;6:e1001110. doi: 10.1371/journal.ppat.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]