Abstract
Background
We assessed the efficacy of serial interferon-gamma release assays (IGRAs) for the diagnosis of latent tuberculosis infection (LTBI) in patients receiving immunosuppressive agents for treatment of rheumatic diseases in Korea.
Methods
Of 276 patients who underwent consecutive screening with one of two IGRAs [QuantiFERON-TB Gold or QuantiFERON-TB Gold In-Tube], 66 patients were evaluated by the serial IGRA for detection of LTBI during therapy with immunosuppressive agents. Information on clinical diagnosis, medication, previous TB, blood cell count, tuberculin skin test, and interferon-gamma (IFN-γ) level measured by IGRA was collected.
Results
Of the 66 patients, the initial IGRA was positive in 24.2%, negative in 65.2%, and indeterminate in 10.6%. Forty-six patients (69.7%) showed consistent IGRA results during follow-up, and 13 patients (19.7%) had consistently positive results. IGRA conversion rate was 12.1% (8/66) and reversion rate was 4.5% (3/66). Conversion of IGRA results was only observed in ankylosing spondylitis patients, and the median interval between the two tests in patients with conversion was 8.5 months. The mean IFN-γ level in the group of patients with consistently positive IGRA results was higher than that in the group with inconsistently positive results, although this trend was not statistically significant (P=0.293). Indeterminate results were observed most frequently in patients with systemic lupus erythematosus.
Conclusions
In patients receiving immunosuppressive agents, both IGRA conversions and reversions were observed. Serial IGRA testing may not be needed in patients with a positive initial IGRA result showing high IFN-γ levels, because of high consistency in the test results.
Keywords: Interferon-gamma release assay, Latent tuberculosis infection, Immunosuppressive agent, Conversion
INTRODUCTION
The vast majority of individuals who are exposed to Mycobacterium tuberculosis do not develop active tuberculosis (TB) disease. Their immune systems are effective in containing the infection as a latent tuberculosis infection (LTBI). However, approximately 10% of individuals with LTBI will develop active TB disease. The risk of developing active TB disease increases in the presence of certain underlying medical conditions (e.g., HIV infection, other types of immunosuppression, and previous history of TB, as determined by chest X-ray) [1, 2]. Diagnosis of LTBI currently depends on tuberculin skin test (TST) reactivity. TST screening is recommended for contacts of TB patients and other groups who are at high risk of development of active TB disease. However, TST has some disadvantages, including the potential for errors due to variability in methods of antigen application and subjective interpretation of results, and low specificity, as it cross-reacts with bacillus Calmette-Guérin (BCG) and nontuberculous environmental mycobacteria [3, 4]. The TST-positive rate (induration diameter ≥10 mm) was 51% in a group of patients ranging in age from 24 to 36 years, and up to 71% in a group having close contact with TB patients in Korea, because BCG vaccination is mandatory in this country, causing high false positive rates [5]. A new test for LTBI, the interferon-gamma release assay (IGRA), has recently been developed, which measures interferon-gamma (IFN-γ) release by T-cells stimulated in vitro with specific mycobacterial antigens. For the ELISA format, the QuantiFERON-TB Gold and QuantiFERON-TB Gold In-Tube tests (QFT-G and QFT-GIT; Cellestis Limited, Carnegie, Victoria, Australia) are approved by the U.S. Food and Drug Administration (FDA). Another format, the enzyme-linked immunospot (ELISPOT) assay (T-SPOT.TB test; Oxford Immunotec Limited, Abingdon, United Kingdom) was approved by the FDA in 2008 [6]. Centers for Disease Control and Prevention (CDC) guidelines suggest that QFT-G can replace the TST under all circumstances in which the TST is currently used, including periodic testing [7]. Updated guidelines on the use of IGRAs were recently published, and it is recommended that an IGRA should be the preferred choice for testing persons who have received BCG as a vaccine. The use of IGRAs in this population is expected to improve diagnostic specificity and encourage acceptance of treatment of LTBI [8]. Investigation of LTBI in patients treated with immunosuppressive agents is important because such patients are at increased risk of LTBI reactivation [9]. For patients with rheumatic disease who have been treated with immunosuppressive agents for a long period of time, monitoring of LTBI is particularly necessary. However, serial IGRA testing of patients treated with immunosuppressive agents has rarely been studied.
In this study, we assessed the performance of IGRA in serial testing for LTBI in rheumatic disease patients treated with immunosuppressive agents in Korea, an intermediate TB burden country. The levels of IFN-γ in patients with positive IGRA results were investigated and IGRA conversions and reversions were evaluated.
MATERIALS AND METHODS
1. Study populations
From September 2006 to November 2010, 276 patients who were consecutively referred by the rheumatology department to the immunology laboratory of Dong-A University Hospital for an IGRA were initially enrolled in this study. Of these, 69 patients (25.0%) had serial IGRA results. A total of 210 specimens were obtained from these 69 patients. Patient charts were reviewed for demographic information, clinical diagnosis, medication information, previous TB history, blood cell count, IFN-γ levels measured by the IGRA assay, and the results of TST. Rheumatic diagnoses were determined by specialist clinicians. Among these 69 patients, 66 patients with rheumatic inflammatory disease who were treated with immunosuppressive agents were included in this study. Three patients, of whom two had fever of unknown origin and one had Kikuchi disease, were not treated with immunosuppressive agents, and therefore were excluded from the study.
2. Interferon-gamma release assay
A total of 66 patients who received immunosuppressive agents were serially screened using the QFT-G assay or QFT-GIT assay. The QFT-G test (Cellestis Limited, Carnegie, Victoria, Australia) was performed according to the manufacturer's recommendations. Briefly, four aliquots of heparinized whole blood were incubated with early secretory antigenic target-6 (ESAT-6), culture filtrate protein 10 (CFP-10), mitogen, and nil (i.e., saline) antigens. Following 12-18 hr of incubation at 37℃ in a humidified atmosphere, the plasma was aspirated from each well, and the amount of IFN-γ was measured by ELISA. For an ELISA run to be valid, the following performance criteria had to be fulfilled: a variation coefficient <15% and a correlation coefficient of the standard curve >0.98. ELISA data were converted to IU/mL using the IFN-γ standard curve generated for each ELISA plate. IFN-γ values were calculated by subtracting the value obtained with nil antigens, with a cutoff value of 0.35 IU/mL according to the manufacturer's instructions. The result of QFT-G was interpreted as indeterminate if the IFN-γ level was <0.35 IU/mL in the antigen stimulated wells and <0.5 IU/mL in the mitogen well, when nil was ≤0.7 IU/mL. When nil was >0.7 IU/mL, the result was indeterminate when the IFN-γ level was <50% of nil in the antigen stimulated wells and there was any response in the mitogen well. In March 2009, when the QFT-GIT assay was developed by the manufacturer, we replaced QFT-G with QFT-GIT (Cellestis Limited, Carnegie, Victoria, Australia). In QFT-GIT, the control material and antigens are contained in the same tubes as are used to collect blood for the test, thus allowing more direct testing of fresh blood. In addition, the TB 7.7 antigen was added to the combination of ESAT-6 and CFP-10 used in QFT-G. To measure the IFN-γ response to TB antigens, the IFN-γ concentration in plasma, from blood stimulated with a single cocktail of peptides representing ESAT-6, CFP-10, and part of TB 7.7, was calculated minus nil. The QFT-GIT test was also performed and interpreted according to the manufacturer's recommendations. The IFN-γ response cutoff value was 0.35 IU/mL as in the QFT-G assay. Results with a nil of 0.7-8.0 IU/mL and an IFN-γ response of 25%-50% of nil in QFT-GIT were interpreted as positive, whereas in QFT-G these results were interpreted as indeterminate. Also, tests with a nil of 0.7-8.0 and an IFN-γ response of <25% of nil were interpreted as negative in QFT-GIT, whereas in QFT-G they were interpreted as indeterminate. Conversion of IGRA was defined as negative IGRA at baseline and positive IGRA at follow-up, and reversion of IGRA was defined as positive IGRA at baseline and negative IGRA at follow-up.
3. Statistical analysis
Statistical analyses were conducted using MedCalc version 9.3 (MedCalc Software, Mariakerke, Belgium). Concordance between serial tests was assessed using kappa (κ) coefficients. The mean levels of IFN-γ in the different patient groups with initially positive IGRA results, and the differences in WBC, neutrophil, and lymphocyte counts between disease groups were compared by Kruskal-Wallis and Mann-Whitney tests. Fisher's exact test was used to compare the frequency of indeterminate results of IGRA according to disease group. P values <0.05 were considered statistically significant.
RESULTS
1. Patients
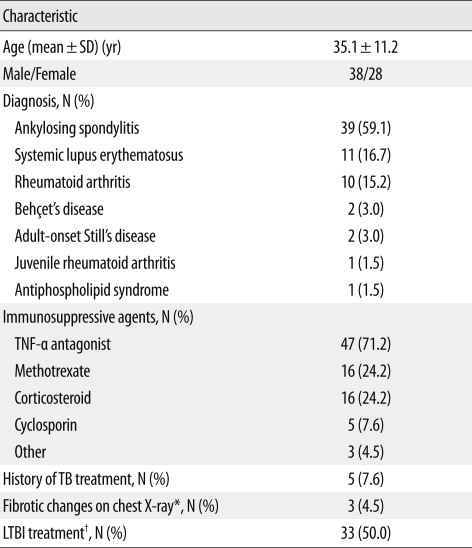
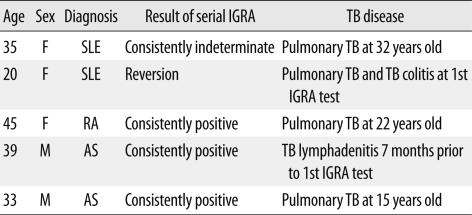
Sixty-six patients who were treated with immunosuppressive agents were serially screened using the IGRA from September 2006 to November 2010. Table 1 shows the characteristics of these patients. Twenty-one patients were treated with combined immunosuppressive agents. Tumor necrosis factor (TNF)-α antagonists were the most commonly used agents. All patients with ankylosing spondylitis (AS), six patients with rheumatoid arthritis (RA), a patient with Behçet's disease, and a patient with juvenile rheumatoid arthritis received TNF-α antagonists. Forty-seven patients (71.2%) were given their initial IGRA test prior to TNF-α antagonist therapy, whereas 19 patients (28.9%) were initially examined by IGRA during their treatment with immunosuppressive agents. In addition, all patients were screened for active TB using chest X-rays and evaluation of clinical symptoms. Of 66 patients, five had a history of TB treatment, which is summarized in Table 2.
Table 1.
Characteristics of the 66 rheumatic disease patients in this study treated with immunosuppressive agents and serially tested by IGRA
*Chest X-ray findings were consistent with previous tuberculosis disease; †Treatment of LTBI was performed based on a positive result from the interferon-gamma release assay and/or a positive response to the tuberculin skin test.
Abbreviations: SD, standard deviation; TNF, tumor necrosis factor; TB, tuberculosis; LTBI; latent tuberculosis infection.
Table 2.
Summary of patients with history of TB treatment
Abbreviations: IGRA, interferon-gamma release assay; TB, tuberculosis; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; AS, ankylosing spondylitis.
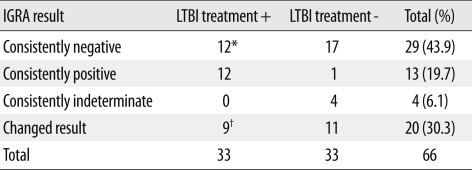
Thirty-three patients (50.0%) were treated for LTBI based on positive IGRA and/or TST results (Table 3). Twelve (36.4%) of the 33 patients were treated based on positive results of TST alone, two patients were retreated for LTBI because IGRA conversion was observed, and one patient was retreated due to an increased TST reaction.
Table 3.
Treatment of latent tuberculosis infection according to distribution of IGRA results
*Twelve patients were treated for LTBI based on the positive tuberculin skin test; †Eight patients with IGRA conversion and one patient with IGRA reversion were included.
Abbreviations: IGRA, interferon-gamma release assay; LTBI, latent tuberculosis infection.
The mean time interval between IGRA tests was 9.2 months (range 1-38), and the mean IGRA test number was 3.1 (range 2-7). Thirty-one (47%) of the 66 patients received only two IGRA tests, and 16 patients (24.2%) received three tests. Two of the AS patients had six consecutive positive IGRA results and seven consecutive negative IGRA results, respectively. According to disease group, the mean number of IGRAs was 3.6 (range 2-7) for AS patients, 2.3 (range 2-3) for RA, 2.4 (range 2-4) for systemic lupus erythematosus (SLE), and 2.3 (range 2-3) for other diseases.
2. Agreement between serial IGRAs and different types of IGRA
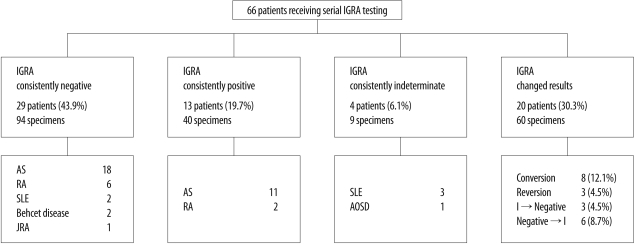
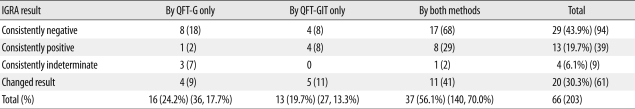
A total of 203 IGRAs were performed. The initial IGRA result was positive in 16 patients (24.2%), negative in 43 (65.2%), and indeterminate in seven (10.6%). Forty-six patients (69.7%) showed consistent IGRA results during follow-up (Fig. 1). Agreement between results of the initial IGRA and those of the second IGRA was 69.7%, with a κ coefficient of 0.460 (95% confidence interval, 0.267-0.653). The percentage of consistent results from patients who had been tested with both QFT-G and QFT-GIT (26/37, 70.3%) was similar to that of patients tested with QFT-G only (12/16, 75.0%) and that of patients tested with QFT-GIT only (8/13, 61.5%) (Table 4).
Fig. 1.
Study flow diagram.
Abbreviations: IGRA, interferon-gamma release assay; AS, ankylosing spondylitis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; JRA, juvenile rheumatoid arthritis; AOSD, adult-onset Still's disease; I, indeterminate IGRA result.
Table 4.
Number of patients according to the results and type of IGRA
Abbreviations: IGRA, interferon-gamma release assay; GFT-G, QuantiFERON-TB Gold test; QFT-GIT, QuantiFERON-TB Gold In-Tube test. Numbers in parentheses are numbers of specimens.
3. Conversion and reversion rates in serial IGRA
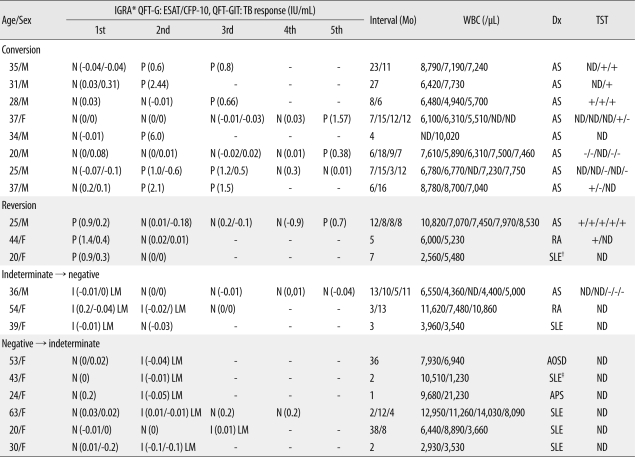
The IGRA conversion rate was 12.1% (8/66) and the reversion rate was 4.5% (3/66). Conversion was only observed in AS patients (8/39, 20.5%). Among patients who received TNF-α antagonists, six of the eight non-AS patients showed consistently negative IGRA results. One patient of the remaining two had consistently positive IGRA results and the other patient had consistently indeterminate results. The median interval between two tests in patients with conversion was 8.5 months (range 6-27). There were two patients who had showed consecutive conversion and reversion during follow-up. One of the eight patients with conversion showed reversion after two consecutive positive results, and one of the three patients with reversion had a second positive result after three consecutive negative results. The mean level of IFN-γ response in the group with consistently positive IGRA results was higher than that in the groups with conversion or reversion, although the differences were not statistically significant (Tables 5 and 6).
Table 5.
Levels of interferon-gamma in patients with initial positive IGRA results
*For the consistently positive and reversion groups, the initial IGRA result was used. For the conversion group, the first positive IGRA result was used. †P=0.293 by Kruskal-Wallis test.
Abbreviations: IGRA, interferon-gamma release assay; IFN-γ, interferon-gamma.
Table 6.
Data on 20 patients with changed IGRA results
*Indicates the amount of IFN-γ in antigen stimulated wells (ESAT-6 and CFP-10 in QFT-G, TB response in QFT-GIT) subtracting the level in the nil well; †The patient was diagnosed with pulmonary TB and TB colitis; ‡The patient had a fibrotic lesion on chest X-ray.
Abbreviations: IGRA, interferon-gamma release assay; GFT-G, QuantiFERON-TB Gold test; QFT-GIT, QuantiFERON-TB Gold In-Tube test; TST, tuberculin skin test; N, negative; P, positive; I, indeterminate; ND, not done; AS, ankylosing spondylitis; TB, tuberculosis; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; AOSD, adult-onset Still's disease; APS, antiphospholipid syndrome; LM, low mitogen.
4. Frequency of indeterminate results according to disease group
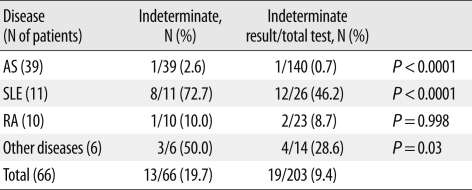
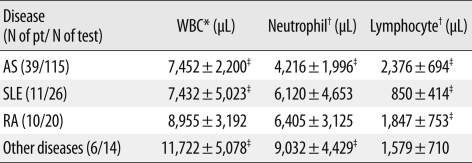
Overall, indeterminate IGRA results were observed in 19/203 tests (9.4%). Of the 66 patients, 13 (19.7%) showed at least one indeterminate result, and four of these 13 patients had consistently indeterminate results. The percentage of indeterminate IGRA results differed significantly according to disease group, and was highest among patients with SLE (8/11, 72.7%) (Table 7). The mean lymphocyte count of SLE patients (850/µL) was the lowest among the disease groups (P<0.001) (Table 8).
Table 7.
Frequency of indeterminate IGRA results according to disease group
The proportion of indeterminate results in total tests was compared with that of all patients by Fisher's exact test.
Abbreviations: IGRA, interferon-gamma release assay; AS, ankylosing spondylitis; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis.
Table 8.
Relationship between blood cell parameters and disease group
Results are expressed as mean±SD.
*P=0.003; †P=0.001 by Kruskal-Wallis test; ‡P<0.001 by Mann-Whitney test.
Abbreviations: pt, patient; AS, ankylosing spondylitis; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis.
DISCUSSION
Investigation of LTBI in patients treated with immunosuppressive agents is important because such patients are at increased risk of LTBI reactivation [9]. Although it is common practice to screen patients for LTBI prior to commencement of TNF-α antagonist therapy [10], guidelines for subsequent monitoring of LTBI in patients receiving immunosuppressive agents, including TNF-α antagonists, have not been established. Monitoring of LTBI risk with IGRA has several advantages, including superior specificity compared to TST, as demonstrated in the case of patients vaccinated with BCG, as well as avoidance of a second visit and subjective variability in TST results [11]. In our study, we assessed the performance of the IGRA in serial testing for LTBI in patients with rheumatic disease undergoing treatment with immunosuppressive agents. Our study shows that the initial results of IGRA are in fair agreement with those of follow-up IGRA during immunosuppressive therapy in a country with an intermediate TB burden. IGRA conversion was observed in eight patients (12.1%) who received TNF-α antagonists, and was only observed in AS patients (20.5%). Among patients who received TNF-α antagonists, six of eight non-AS patients showed consistently negative IGRA results, and the remaining two patients had consistently positive results and consistently indeterminate results, respectively. The reason for the occurrence of IGRA conversion only in AS patients is uncertain, although a previous study reported that the TST conversion rate was significantly higher in AS patients than in RA patients, when both groups received TNF-α antagonists [12]. In 27 RA patients in Taiwan who had completed a 12-month period of therapy with TNF-α antagonists, conversion of QFT-G was not observed [13]. In our study, the median interval between two tests in patients with conversion was 8.5 months, which is a relatively short period. Further studies are needed to establish the most appropriate follow-up interval for IGRA in patients treated with TNF-α antagonists. The conversion rate of IGRA may differ according to disease group and duration of follow-up. Further studies would also clarify the factors affecting IGRA conversion.
Among patients with positive IGRA results, nearly 20% (13/66, 19.7%) had consistently positive results. The mean level of IFN-γ response in consistently IGRA-positive patients was higher than that in patients with IGRA reversion, indicating that results showing a low positive IFN-γ response tend to be inconsistent. Two (3.0%) of the 66 patients showed both conversion and reversion in our study. Similar results were reported from a study of the reproducibility of QFT-GIT in a targeted US screening population. In that study, transient responses to QFT-GIT were common; therefore, it was concluded that low positive IFN-γ results (<0.69 IU/mL) should be interpreted with caution [14]. In our study, a SLE patient who showed a low IFN-γ response for ESAT-6 only was diagnosed as having active pulmonary TB and TB colitis, and the result of QFT-G was found to be negative 18 months later. In a study by Chen et al. [13], two patients who showed reversion on the QFT-G test had clinical TB diseases, such as TB arthritis and TB pleurisy. The clinical significance of reversion may be different in cases of transient responses to contact infection, and in patients who have received immunosuppressive agents. Further study is needed to define the clinical significance of IGRA reversion.
Traditionally, TST was the only practical immunological test for the diagnosis of LTBI before the development of IGRA. In this study, 12 (36.4%) of the 33 patients who received treatment for LTBI were treated based on positive TST results only. A similar result was reported in a study from Korea. Of 28 converted TST-positive immune mediated inflammatory disease patients who had received TNF-α antagonists, 16 patients were examined by IGRA, and only five results were positive [12]. These data indicate that positive TST is more prevalent than positive IGRA among patients who have received TNF-α antagonists in Korea. These discrepancies may be explained by a cross-reaction with nontuberculous mycobacteria in TST and false negative results of IGRA [15, 16]. Further study is needed to establish optimal screening guidelines for LTBI in patients who have received TNF-α antagonists in areas with mandatory BCG vaccination.
Four (6.1%) of 66 rheumatic disease patients who received immunosuppressive agents showed continuously indeterminate GFT-G results. Published data on the overall rate of indeterminate results from patients with rheumatic diseases are comparable to our results, ranging from 1.9% to 6% [17-19]. In contrast, a high rate of indeterminate QFT-G test results (28.6%) was previously reported amongst RA patients [20]. Although patients with indeterminate results are reported as having a statistically significant change in test results upon follow-up QFT-G (23%) [18], only three patients (4.5%) showed a change from indeterminate results in the present study.
In our study, indeterminate results were observed most frequently in patients with systemic lupus erythematosus. Kobashi et al. reported that four of five active TB patients with indeterminate QFT-TB (corresponding to QFT-G) results had moderate lymphocytopenia [21]. In another recent study, the sensitivity of IGRA partly depended on peripheral lymphocyte counts and under low lymphocyte count conditions, ELISPOT was superior to QFT-G for detecting TB [22]. Lymphocytopenia is a frequent finding in SLE patients. In the present study, 8 of 11 SLE patients showed at least one indeterminate response during the follow-up period, and lymphocytopenia was observed only in SLE patients. Although the number of patients in our study is limited, these observations suggest that the results of IGRA may be influenced by cellular factors of the host.
In conclusion, conversions and reversions were observed in patients receiving immunosuppressive agents. Serial IGRA testing may not be needed in patients with positive initial IGRA results showing high IFN-γ levels, because of the consistency in the results of IGRA over a considerable period of time. The relationship between the magnitude of change in IFN-γ level, as measured by IGRA, and conversion and reversion rates requires further clarification.
Acknowledgements
This study was supported by research funds from Dong-A University.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Small PM, Fujiwara PI. Management of tuberculosis in the United States. N Engl J Med. 2001;345:189–200. doi: 10.1056/NEJM200107193450307. [DOI] [PubMed] [Google Scholar]
- 2.Horsburgh CR., Jr Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060–2067. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 3.Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med. 2002;347:1860–1866. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804–809. doi: 10.1136/thorax.57.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–2761. doi: 10.1001/jama.293.22.2756. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration, editors. T-SPOT-TB-P070006. [Updated on Jul 2008]. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P070006. [Google Scholar]
- 7.Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB gold test for detecting mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55. [PubMed] [Google Scholar]
- 8.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 9.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 10.Korea Food and Drug Administration. Guideline for diagnosis and treatment of latent tuberculosis infection in patients treated with TNF blockers. Korea Food and Drug Administration; 2004. [Google Scholar]
- 11.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH, Seo GY, Lee JS, Kim TH, Yoo DH. Positive conversion of tuberculin skin test and performance of interferon release assay to detect hidden tuberculosis infection during anti-tumor necrosis factor agent trial. J Rheumatol. 2009;36:2158–2163. doi: 10.3899/jrheum.090150. [DOI] [PubMed] [Google Scholar]
- 13.Chen DY, Shen GH, Hsieh TY, Hsieh CW, Lan JL. Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum. 2008;59:800–806. doi: 10.1002/art.23705. [DOI] [PubMed] [Google Scholar]
- 14.Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB gold in-tube assay. Clin Vaccine Immunol. 2008;15:425–432. doi: 10.1128/CVI.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005;20:913–925. doi: 10.3346/jkms.2005.20.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdi H, Mariette X, Godot V, Weldingh K, Hamid AM, Prejean MV, et al. Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Res Ther. 2006;8:R114. doi: 10.1186/ar1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matulis G, Jüni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 18.Inanc N, Aydin SZ, Karakurt S, Atagunduz P, Yavuz S, Direskeneli H. Agreement between Quantiferon-TB gold test and tuberculin skin test in the identification of latent tuberculosis infection in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2009;36:2675–2681. doi: 10.3899/jrheum.090268. [DOI] [PubMed] [Google Scholar]
- 19.Malaviya AN, Kapoor S, Garg S, Rawat R, Shankar S, Nagpal S, et al. Preventing tuberculosis flare in patients with inflammatory rheumatic diseases receiving tumor necrosis factor-alpha inhibitors in India -- An audit report. J Rheumatol. 2009;36:1414–1420. doi: 10.3899/jrheum.081042. [DOI] [PubMed] [Google Scholar]
- 20.Shovman O, Anouk M, Vinnitsky N, Arad U, Paran D, Litinsky I, et al. QuantiFERON-TB gold in the identification of latent tuberculosis infection in rheumatoid arthritis: a pilot study. Int J Tuberc Lung Dis. 2009;13:1427–1432. [PubMed] [Google Scholar]
- 21.Kobashi Y, Obase Y, Fukuda M, Yoshida K, Miyashita N, Oka M. Clinical reevaluation of the QuantiFERON TB-2G test as a diagnostic method for differentiating active tuberculosis from nontuberculous mycobacteriosis. Clin Infect Dis. 2006;43:1540–1546. doi: 10.1086/509327. [DOI] [PubMed] [Google Scholar]
- 22.Komiya K, Ariga H, Nagai H, Teramoto S, Kurashima A, Shoji S, et al. Impact of peripheral lymphocyte count on the sensitivity of 2 IFN-gamma release assays, QFT-G and ELISPOT, in patients with pulmonary tuberculosis. Intern Med. 2010;49:1849–1855. doi: 10.2169/internalmedicine.49.3659. [DOI] [PubMed] [Google Scholar]