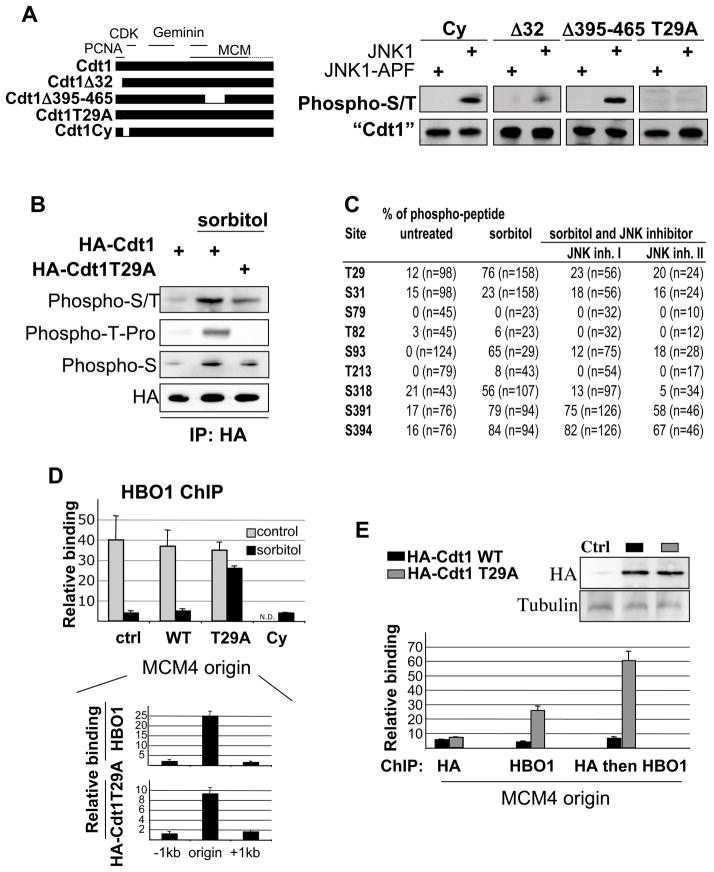
Figure 5. Cdt1 phosphorylation impairs HBO1 binding at origins.
(A) Phosphorylation of the indicated Cdt1 derivatives co-transfected with JNK1 or kinase-deficient JNK1 in HeLa cells. Description of the different Cdt1 variants is indicated on the left with interaction domains with PCNA, CDK, Geminin and MCM highlighted.
(B) Western blot analysis of Cdt1 and Cdt1 T29A phosphorylation under osmotic stress conditions with a context-specific phospho-T antibody.
(C) Cdt1 phosphorylation sites induced by JNK1 and sorbitol. Table represent the percentage of phospho-peptide for each serine and threonine residue recovered over the 3 samples. The total number of peptide for each site is indicated into bracket.
(D) HBO1 association at the MCM4 origin in HeLa cells transfected with the indicated Cdt1 derivatives and subjected or not to sorbitol treatment (ND, not determined). Bottom panel: Association of HBO1 and HA-Cdt1-T29A at the MCM4 origin and surrounding regions in cells subject to sorbitol treatment.
(E) Sequential ChIP analysis of HBO1+HA-Cdt1 (black) or HBO1+HA-Cdt1-T29A (grey) binding at the MCM4 replication origin in HeLa cells treated with sorbitol. Top panel: Western blot analysis of HA-fusions expression.