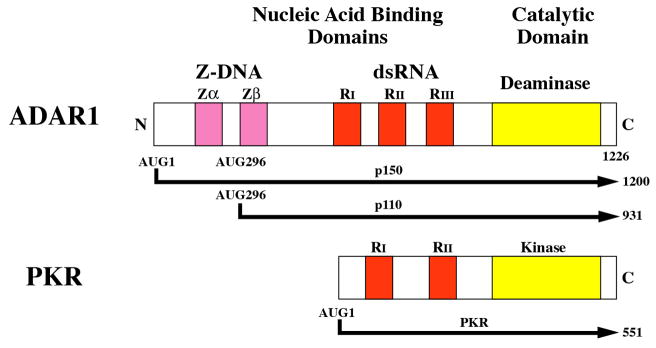
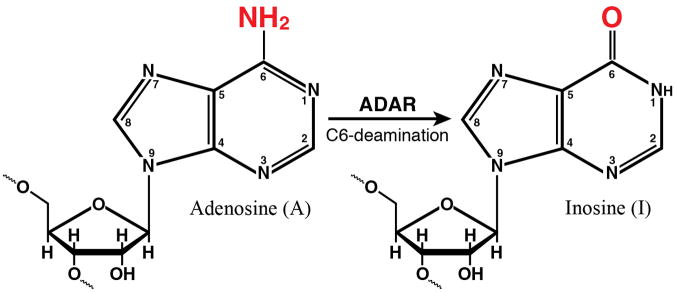
Figure 2. Domain organization of ADAR1 and PKR proteins from human cells and the enzymatic reactions catalyzed by them.
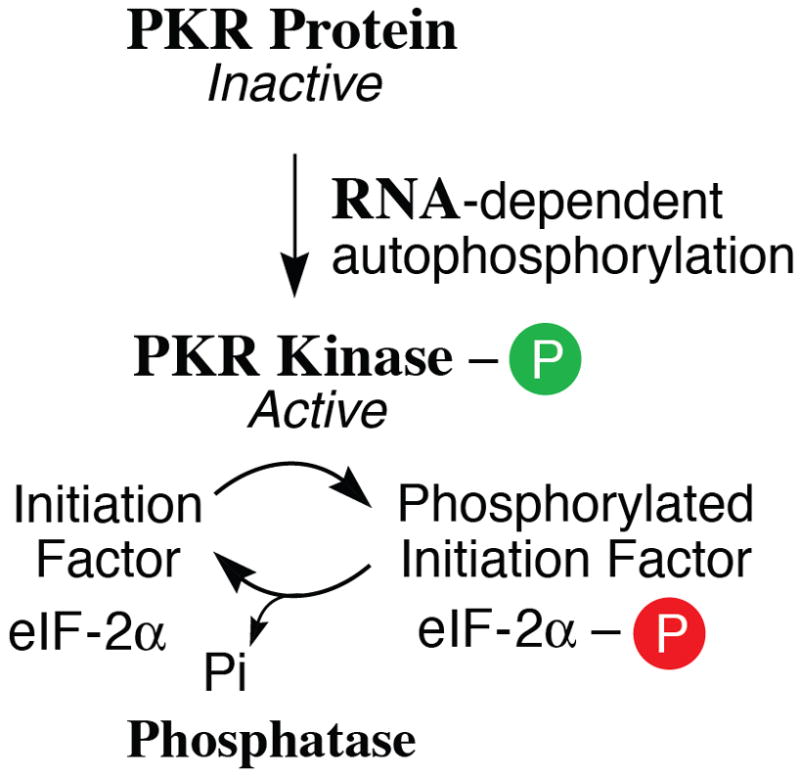
(A) Domains. Alternative promoters and alternative splicing give rise to two size isoforms of ADAR1, an IFN-inducible p150 protein and a constitutively expressed p110 protein. A single-sized form of PKR is known that is inducible by IFN. The N-terminal regions of ADAR1 and PKR include repeated nucleic acid binding domains and the C-terminal regions the catalytic domains responsible for their enzymatic activities. Multiple dsRNA binding domains (RI, RII, RIII), three present in both ADAR1 p110 and p150 and two in PKR, are shown in red. RNA adenosine deaminase and protein kinase catalytic domains are shown in yellow for ADAR1 and PKR, respectively. The N-terminal region of the p150 form of ADAR1 also possesses two Z-DNA binding domains (Zα and Zβ, and p110 the Zβ copy, as shown in pink. (B) dsRNA is the substrate of ADAR1. ADAR1 p110 and p150 catalyze the C-6 deamination of adenosine (A) to yield inosine (I) in RNA with double-stranded character. (C) dsRNA is an effector of PKR. RNA-dependent activation of eIF2α protein kinase activity is mediated by autophosphorylation (green P) of PKR; phosphorylation of serine 51 (red P) of eIF2α by PKR leads to an inhibition of protein synthesis.