Abstract
Objective: To investigate the role of iptakalim, an ATP-sensitive potassium channel opener, in transient cerebral ischemia/reperfusion (I/R) injury and its involved mechanisms. Methods: Intraluminal occlusion of middle cerebral artery (MCAO) in a rat model was used to investigate the effect of iptakalim at different time points. Infarct volume was measured by staining with 2,3,5-triphenyltetrazolium chloride, and immunohistochemistry was used to evaluate the expressions of Bcl-2 and Bax. In vitro, neurovascular unit (NVU) cells, including rat primary cortical neurons, astrocytes, and cerebral microvascular endothelial cells, were cultured and underwent oxygen-glucose deprivation (OGD). The protective effect of iptakalim on NVU cells was investigated by cell viability and injury assessments, which were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and release of lactate dehydrogenase. Caspase-3, Bcl-2 and Bax mRNA expressions were evaluated by real-time polymerase chain reaction (PCR). Results: Administration of iptakalim 0 or 1 h after reperfusion significantly reduced infarct volumes, improved neurological scores, and attenuated brain edema after cerebral I/R injury. Iptakalim treatment (0 h after reperfusion) also reduced caspase-3 expression and increased the ratio of Bcl-2 to Bax by immunohistochemistry. Iptakalim inhibited OGD-induced cell death in cultured neurons and astrocytes, and lactate dehydrogenase release from cerebral microvascular endothelial cells. Iptakalim reduced mRNA expression of caspase-3 and increased the ratio of Bcl-2 to Bax in NVU cells. Conclusions: Iptakalim confers neuroprotection against cerebral I/R injury by protecting NVU cells via inhibiting of apoptosis.
Keywords: Neurovascular unit, Cerebral ischemia/reperfusion (I/R) injury, ATP-sensitive potassium channel opener, Neuroprotection, Apoptosis
1. Introduction
Morbidity from reperfusion and postischemia damages remains common despite improvements in imaging, interventional techniques, and pharmacolo-gical agents. Cerebral ischemia/reperfusion (I/R) injury activates multiple and distinct, but overlapping, cell signaling pathways, which may cause cell death (Linnik et al., 1993). Reperfusion injury is a paradoxical and complex phenomenon that may promote free radicals (Chan, 1996), cell damage, and cell death (Graham and Chen, 2001).
In the past, most studies on brain I/R injury emphasized effects on neurons, and were unsuccessful in fully elucidating the mechanism responsible for brain damage in I/R. The neurovascular unit (NVU), a functional module composed of neurons, astrocytes, and endothelial cells, has played an important role in understanding cerebrovascular diseases (Lo and Rosenberg, 2009). Elucidation of the role of NVU in various types of cells may facilitate the development of novel therapies for stroke.
Although the ATP-sensitive potassium (KATP) channel is extensively expressed in cerebral cortex, hippocampus, hypothalamus, and substantia nigra pars compacta, the molecular components of the neuronal KATP channels do not appear to be homogeneous (Dunn-Meynell et al., 1998). KATP channels are also present in astrocytes (Simard and Chen, 2004) and endothelial cells (Janigro et al., 1993; Mederos y Schnitzler et al., 2000; Yoshida et al., 2004).
Iptakalim, a unique type of KATP channel opener, exerts its neuroprotective effects by activating the sulfonylurea receptor 2 (SUR2), rather than the SUR1, type of KATP channel, and is a promising antihypertensive drug for clinical use (Chen et al., 2004; 2006; Wang et al., 2007). In addition, the whole cell patch clamp technique showed that iptakalim has a high affinity for neuronal KATP channels in the brain (Wang et al., 2004). However, the effects of iptakalim on cerebral I/R injury and NVU cells are unknown.
We used a rat model of transient cerebral I/R that mimics the postevent injury using middle cerebral artery occlusion (MCAO). We tested the hypothesis that pharmacological treatment with iptakalim confers neuroprotection against cerebral I/R injury by protecting NVU cells and investigated the underlying mechanism.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats, weighing 250 to 260 g, were purchased from the Experimental Animal Centre (Beijing, China). All the work described has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, and European Communities Council Directive 86/609/EEC for animal experiment, and was approved by the Local Ethical Committee on Animal Studies and the Academy of Military Medical Sciences, Beijing, China.
2.2. Chemical compound
Iptakalim was synthesized by Thadweik Academy of Medicine, Beijing, China. All other materials and chemicals were purchased from commercial resources.
2.3. MCAO reperfusion model
MCAO was induced as described with minor modification (Yanamoto et al., 1998). Briefly, after exposing and opening the right common carotid artery, a 6-0 nylon monofilament (Ethilon, Ethicon Inc.) coated with silicon resin (Heraeus) was advanced until a faint resistance. The nylon thread was withdrawn after occlusion for 120 min, and then reperfusion was achieved. A heating blanket was used to maintain rat body temperature at 36.5 to 37.5 °C during the surgery and anesthesia.
2.4. Experimental groups
In the previous study, 8 mg/kg BW of iptakalim had a protective effect on brain edema caused by acute hypobaric hypoxia in rats (Zhu et al., 2008). So 8 mg/kg BW of iptakalim was administrated by oral once per day for 3 d after I/R. According to the different time points administering iptakalim for the first time, animals were randomly divided into different treatment groups, as shown in the Table 1.
Table 1.
Treatment protocols
| Group | Treatment | Administration time |
| Sham | Saline 0.5 ml/100 g BW | 0 h post reperfusion |
| I/R control | Saline 0.5 ml/100 g BW | MCAO for 120 min reperfusion |
| I/R+Ipt | Ipt 8 mg/kg BW | 1 h pre-ischemia |
| I/R+Ipt | Ipt 8 mg/kg BW | 1 h pre-reperfusion |
| I/R+Ipt | Ipt 8 mg/kg BW | 0 h post reperfusion |
| I/R+Ipt | Ipt 8 mg/kg BW | 1 h post reperfusion |
| I/R+Ipt | Ipt 8 mg/kg BW | 2 h post reperfusion |
| I/R+Ipt | Ipt 8 mg/kg BW | 4 h post reperfusion |
I/R: ischemia/reperfusion; Ipt: iptakalim; BW: body weight
2.5. Determination of neurological deficit scores
Neurological deficits were evaluated 70 h after MCAO according to the five-point scale (Yang and Betz, 1994). Score 5 indicates the worst damage and represents death deficit in this system.
2.6. Determination of infarct volume
After neurological measurement of the rat, brain tissues were sliced at a section thickness of 1 mm. Slices were incubated in 0.1% (v/v) 2,3,5-triphenyl-tetrazolium chloride (TTC) solution for 30 min at 37 °C. Samples were then fixed in 10% (v/v) phosphate-buffered formalin and photographed. Using a computerized image analysis system (Image Pro Plus 6.0; Media Cybernetics), the infarct area in each sample was measured. The percentage of hemispheric infarction volume was calculated as described by previous studies (Swanson and Sharp, 1994; Roof et al., 2001).
2.7. Detection of brain edema
For evaluation of brain water content, brains were removed quickly after 70 h of reperfusion. The wet weigh (WW) and dry weight (DW) were obtained before and after drying brain tissues in an oven at 95 °C for 24 h, respectively. The percent water content (WC) was calculated using the formula (Zhu et al., 2008): WC (%)=(WW−DW)×100%/WW.
2.8. Immunohistochemistry for markers of apoptosis
The animals were decapitated 70 h after reperfusion, and brains were removed and fixed in 10% (v/v) buffered formaldehyde. Brain samples (ipsilateral MCAO) were embedded in paraffin. Sections (7 μm thick) were incubated in 10% (v/v) normal goat serum (Boster Biotech Ltd., Wuhan, China) in 10 mmol/L phosphate-buffered saline (PBS) for 120 min before incubation with monoclonal antibody cleaved caspase-3 (1:200; Boster Biotech Ltd.) overnight. After rinsing the sections in neopentylglycol succinate, endogenous peroxidase was blocked by incubating the sections for 30 min with 3% (v/v) H2O2 and 10% (v/v) methanol in PBS. After washing, sections were incubated for 2 h in biotinylated goat anti-mouse IgG (1:1000; Boster Biotech Ltd.) in PBS. Sections were then washed, and avidin-biotin complex (ABC) reagent (Boster Biotech Ltd.) was added and incubated for 30 min. After the incubation, diaminobenzidine was added and incubated for 5 min, and slides were dehydrated, dried, and covered with a coverslip. The same methods were applied using Bcl-2 antibody (1:200; Boster Biotech Ltd.) or Bax antibody (1:200; Boster Biotech Ltd.) to determine their protein expression in tissues. Apoptosis was quantified using relative optical density and Image Pro Plus 6.0 software (Media Cybernetics, USA).
2.9. NVU cell models of hypoxic injury
Cultures of rat primary cortical neurons were prepared as described by Abdel-Hamid and Baimbridge (1997). Briefly, one-day-old Sprague-Dawley rats were anesthetized, decapitated, and sterilized in 95% (v/v) ethanol for 1 min. Cortical tissue was dissected, placed in PBS, cut into small pieces, and then incubated with 2.5 g/L trypsin for 10 min at 37 °C. Cell suspension was centrifuged at 800 r/min at room temperature. The pellets were then resuspended, and cells were plated at a density of 3×105 cells/cm2 on poly-d-lysine-coated coverslips in Dulbecco’s modified Eagle medium (DMEM)/F-12 supplemented with 10% (v/v) fetal bovine serum (FBS). After 24-h incubation, the medium was replaced with Neurobasal medium containing 1% (v/v) B27 supplement, 100 U/ml penicillin, and 100 mg/ml streptomycin. Cytosine arabinoside (10 μmol/L) was added after a 48-h incubation to inhibit glial cell proliferation. After 8–10 d of culture, the neurons were >95% positive for neuron-specific enolase, as evaluated by immunocytochemistry (data not shown).
Rat primary astrocytes were prepared as described by Hamprecht and Löffler (1985). Briefly, whole brain tissues of one-day-old Sprague-Dawley rats were triturated, and then cells were plated on poly-d-lysine-coated cell culture flasks in DMEM containing 10% (v/v) FBS, 100 U/ml penicillin, and 100 g/ml streptomycin. Cultures were maintained at 37 °C in a 5% CO2/95% air incubator until 80% confluency was reached (10–14 d of culture). More than 95% of the enriched astrocyte culture was positive for glial fibrillary acidic protein, as measured by immunocytochemistry (data not shown).
Rat cerebral microvascular endothelial cells (CMVECs) were isolated from Sprague-Dawley rats (two-week-old) as described previously (Kis et al., 2002; 2005; Domoki et al., 2005). Briefly, the brain cortices were homogenized and then centrifuged at 1 800 r/m for 6 min in DMEM. The pellets were resuspended in 0.20 g/ml BSA and centrifuged at 1 000×g for 20 min to yield cortical microvessels. The pelleted microvessels were washed in DMEM and then centrifuged at 1 000×g for 10 min in 33% (v/v) Percoll gradient. The band of CMVECs was aspirated and then seeded onto collagen IV-coated cell culture ware after washing twice in DMEM. The culture medium consisted of DMEM supplemented with 2 mmol/L glutamine, 20% (v/v) FBS, 1 ng/ml basic fibroblast growth factor, 100 μg/ml heparin, 50 μg/ml endothelial cell growth supplement, and antibiotics. The 80% confluency was reached (4–5 d of culture). More than 95% enriched CMVECs culture was positive for von Willebrand factor, and negative for glial fibrillary acidic protein and α-smooth muscle actin by immunocytochemistry (data not shown).
We used a hypoxia model, as per a previously described method (Diarra et al., 1999) with minor modification. After removing the growth medium from cultures, the cells were rinsed gently by immersing three times in Earle’s solution (143 mmol/L NaCl, 5.4 mmol/L KCl, 1.8 mmol/L CaCl2, 1 mmol/L MgSO4, 1 mmol/L NaH2PO4, 2.4 mmol/L 4-(2-hydro-xyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4). Fresh Earle’s solution was then added together with 0.5 mmol/L sodium dithionite (the hypoxic buffer) without glucose, and the cultures were immediately cultured at 37 °C in 5% CO2 /95% air for 4 h. Cells were then washed by gently immersing in Earle’s solution and returned to their original growth medium with or without iptakalim (0.01, 0.1, 1 μmol/L) for another 24-h culture. Iptakalim-treated cells were washed with iced PBS before the next measurement.
2.10. Determination of cell injury and viability
After drug treatment, culture medium was replaced with 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma) in fresh medium. Cultures were incubated at 37 °C for 4 h. After discarding the supernatants, 150 μl of dimethyl sulfoxide was added. Absorbance was measured using a Model 550 microplate reader (Bio-Rad, USA) at 570 nm. Cell viability was calculated by dividing the number of cells reduced by MTT by the total number of cells examined, and then multiplying the result by 100%.
The injury effects of anoxia and hypoglycemia on cells were measured by the release of lactate dehydrogenase (LDH) into the incubation medium by LDH detection kit (Nanjing Jiancheng Institute of Biotechnology).
2.11. Real-time quantitative polymerase chain reaction (PCR) to measure mRNA expression
Total cellular RNA was extracted using TRIzol reagent (Invitrogen, USA), and RNA concentrations were determined using a spectrophotometer. One microgram per sample of total RNA was reverse transcribed with 200 U of Superscript II RT (Invitrogen, USA) at 42 °C for 50 min and primed with 0.5 μg of random hexamers. Reverse transcription products were then digested with RNase at 37 °C for 30 min to remove the RNA template from the cDNA:RNA hybrid molecule. One microliter per sample of cDNA was used for PCR amplification using the specific primer pairs as shown in Table 2.
Table 2.
Primers for PCR amplification
| Gene | Primer sequence |
| Bcl-2 | F: 5′-CTCGTCGCTACCGTCGTGACTTCG-3′ |
| R: 5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ | |
| Bax | F: 5′-ACCAGCTCTGAACAGATCATG-3′ |
| R: 5′-TGGTCTTGGATCCAGACAAG-3′ | |
| Caspase-3 | F: 5′-GGTATTGAGACAGACAGTGG-3′ |
| R: 5′-CATGGGATCTGTTTCTTTGC-3′ | |
| β-actin | F: 5′-GGGCACAGTGTGGGTGAC-3′ |
| R: 5′-CTGGCACCACACCTTCTAC-3′ |
F: forward; R: reverse
Quantitative PCR analysis was performed with a SYBRGreen qPCR kit (TianGen) with 3 μl per sample of cDNA and 5 pmol per sample of primers for caspase-3, Bax, Bcl-2, or β-actin. PCR was performed by denaturing at 94 °C for 10 s, annealing at 60 °C (Bax), 58 °C (caspase-3), 60 °C (Bcl-2 ) and 55 °C (β-actin) for 10 s, and extension at 72 °C for 10 s for 39 cycles (β-actin) and 50 cycles (caspase-3, Bcl-2, and Bax, respectively). The final extension was at 72 °C for 10 min. A standard curve was generated using IQ5 optical system software Version 2.0 (Bio-Rad). All experiments were performed in triplicate.
2.12. Statistical analysis
Data for the in vivo study are expressed as the mean±standard deviation (SD), and data for the in vitro study are represented as the mean±standard error of the mean SEM. The Kolmogorov-Smirnov test was used to test for a normal distribution. Group means were compared by one-way analysis of variance (ANOVA) and post-hoc Student-Newman-Keuls test. Differences were considered significant when probability (P) values were less than 0.05.
3. Results
3.1. Iptakalim reduces infarct volume
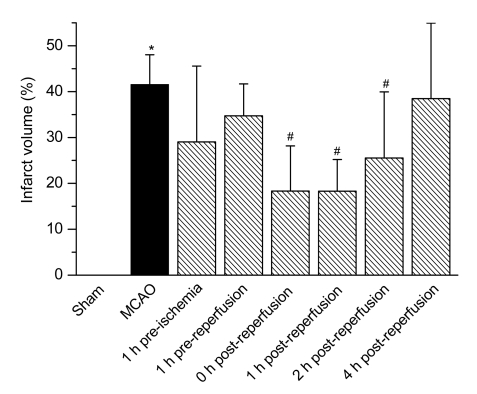
A statistically significant decrease in the infarct volume was observed at 0, 1, and 2 h after reperfusion in MCAO animals that were treated with iptakalim compared with MCAO animals that were treated with saline (Fig. 1). Infarct volumes in MCAO animals that were treated with iptakalim 0 h [(18.33±9.84)%], 1 h [(18.30±6.89)%], and 2 h [(25.52±8.45)%] after reperfusion were less (P<0.05) than that of saline-treated rats receiving MCAO [(41.58±6.47)%]. There were no infarcts in saline-treated rats that received sham treatment.
Fig. 1.
Effects of iptakalim on infarct volume after cerebral I/R injury
Rats were treated with saline (sham operation) or received MCAO. Iptakalim (8 mg/kg BW) was administered to separate groups of rats that received MCAO at 1 h pre-ischemia, 1 h pre-reperfusion, 0, 1, 2, and 4 h post-reperfusion. Data are presented as the mean±SD (n=8). * P<0.05 for rats that received MCAO compared to the sham control group; # P<0.05 for rats that received MCAO plus iptakalim compared with rats that received MCAO plus saline
3.2. Iptakalim improves neurological deficits after I/R injury
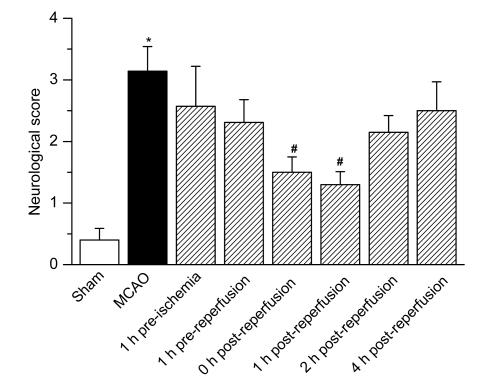
At 0 and 1 h after reperfusion, neurological scores for rats treated with iptakalim were 1.50±0.25 and 1.30±0.21, respectively, which differed significantly from that of saline-treated rats that received MCAO (3.14±0.40), as shown in Fig. 2. Saline-treated MCAO sham rats did not show any remarkable neurological deficit.
Fig. 2.
Effects of iptakalim on neurological deficit scores after cerebral I/R injury
Rats were treated with saline (sham operation) or received MCAO. Iptakalim (8 mg/kg BW) was administered to separate groups of rats that received MCAO at 1 h pre-ischemia, 1 h pre-reperfusion, 0, 1, 2, and 4 h post-reperfusion. Data are presented as the mean±SD (n=8). * P<0.05 for rats that received MCAO compared to the sham control group; # P<0.05 for rats that received MCAO plus iptakalim compared with rats that received MCAO plus saline
3.3. Iptakalim ameliorates brain edema
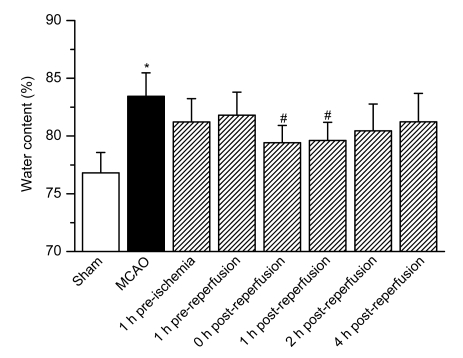
At 0 and 1 h after reperfusion, the brain water contents of rats that were treated with iptakalim were (79.42±1.49)% and (79.61±1.58)%, respectively, which differed significantly from that of saline-treated rats that received MCAO [(83.43±2.04)%], as shown in Fig. 3. Treatment with iptakalim before reperfusion caused only a minor decrease in water content. Saline-treated sham rats did not show any significant difference compared with the iptakalim treatment groups.
Fig. 3.
Effects of iptakalim on brain edema after cerebral I/R injury
Rats were treated with saline (sham operation) or received MCAO. Iptakalim (8 mg/kg BW) was administered to separate groups of rats that received MCAO at 1 h pre-ischemia, 1 h pre-reperfusion, 0, 1, 2, and 4 h post-reperfusion. Data are presented as the mean±SD (n=8). * P<0.05 for rats that received MCAO compared to the sham control group; # P<0.05 for rats that received MCAO plus iptakalim compared with rats that received MCAO plus saline
3.4. Iptakalim inhibits hypoxia-induced cell death in vitro
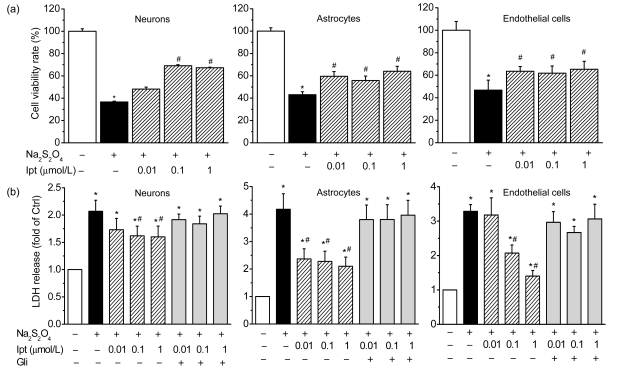
Cells from normal rats were used for primary cultures of cerebrocortical neurons, astrocytes, and endothelial cells. Cell viability was assessed after 24 h of treatment with different concentrations of iptakalim under hypoxic conditions in vitro. Cells were also cultured under nonhypoxic conditions as control and were considered to have a survival rate of 100%. Hypoxia reduced neuronal cell survival to less than 40% of that of the control (Fig. 4a). Treatment with 0.01, 0.1, or 1 μmol/L iptakalim inhibited hypoxia-induced cell death in a concentration-dependent manner. Similar results were obtained for astrocytes and endothelial cells. Treatment with iptakalim at a concentration of 0.01 μmol/L significantly inhibited the hypoxia-induced death of astrocytes and endothelial cells (Fig. 4a).
Fig. 4.
Effects of iptakalim (Ipt; 0.01, 0.1, and 1 μmol/L) on cell death (a) and LDH release (b) in cultures of primary cerebrocortical neurons, astrocytes, and microvascular endothelial cells under hypoxic conditions
Hypoxic conditions were induced using 0.5 mmol/L Na2S2O4. Glibenclamide (Gli, 1 μmol/L) was added to reverse the effects of iptakalim. Data are presented as mean±SEM of four individual experiments. * P<0.05 for cells exposed to hypoxia compared with the normal control group; # P<0.05 for cells that treated with iptakalim under hypoxic condition compared with cells treated with hypoxia without iptakalim
3.5. Iptakalim inhibits hypoxia-induced release of LDH in vitro
To confirm the protective effect of iptakalim under hypoxic conditions in vitro, cell toxicity was assessed by measuring LDH release into the culture medium. Neurons, astrocytes, and endothelial cells were cultured under hypoxic conditions for 24 h. LDH release from all three cell types was significantly higher for cells that were treated with iptakalim than for cells that were treated with saline (P<0.05). Treatment with 0.01, 0.1, or 1 μmol/L iptakalim significantly decreased LDH release from all three cell types compared with untreated cells cultured under hypoxic conditions (P<0.05; Fig. 4b). Glibenclamide, a KATP channel blocker, at a concentration of 1 μmol/L reversed the protective effects of iptakalim in the three cell types. These results suggested that iptakalim protected cells from death by activating KATP channels.
3.6. Iptakalim inhibits apoptosis in vivo and in vitro
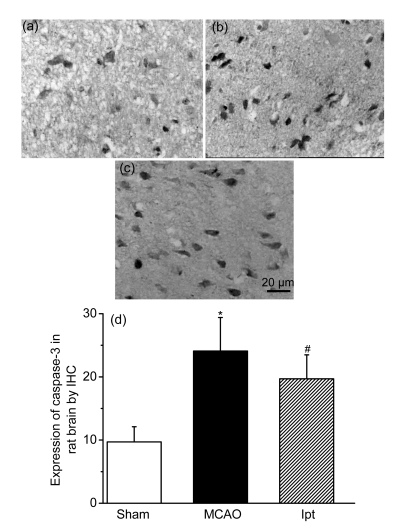
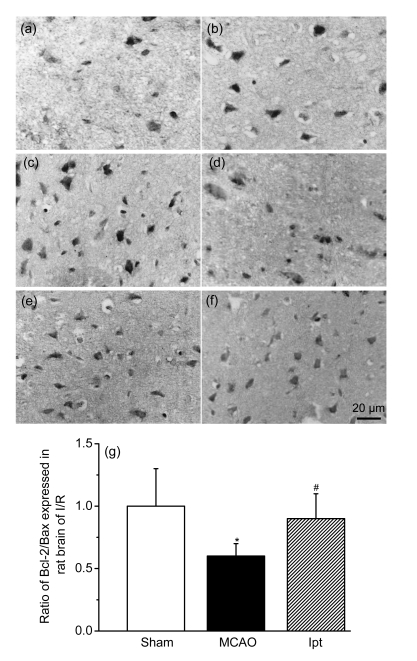
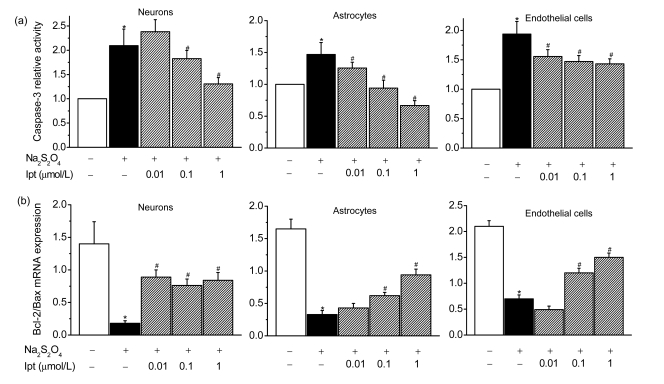
Compared with the sham group, cleaved caspase-3 activity in the cortex (ipsilateral MCAO) was enhanced in the I/R group. Treatment with iptakalim (8 mg/kg BW, 0 h after reperfusion) reduced caspase-3 protein levels compared with levels observed in cells from untreated rats that received I/R (Fig. 5). Levels of Bcl-2, which protects against apoptosis, and Bax, which initiates apoptosis, were also measured. The ratio of the protein level of Bcl-2 to Bax is used to measure the balance between cell survival and cell death (Green and Reed, 1998). Compared with the sham group, the ratio of Bcl-2 to Bax was reduced in the I/R group. Treatment with iptakalim 1 h after reperfusion significantly increased the Bcl-2 to Bax ratio compared with that observed in the I/R group (Fig. 6), suggesting that iptakalim inhibits cerebral apoptosis after cerebral I/R injury. To confirm the antiapoptotic effects of iptakalim, mRNA expressions of caspase-3, Bcl-2, and Bax in NVU cells were measured using real-time quantitative PCR. Caspase-3 mRNA expression was increased in neurons cultured under hypoxic conditions compared to neurons cultured under control conditions. Treatment with 0.01 μmol/L iptakalim reversed the increase in caspase-3 mRNA in neurons cultured under hypoxic conditions (Fig. 7a). The ratio of Bcl-2 to Bax mRNA was reduced in neurons cultured under hypoxic conditions. Iptakalim attenuated the decrease in the ratio of Bcl-2 to Bax mRNA in neurons cultured under hypoxic conditions (Fig. 7b). mRNA expressions of caspase-3 and Bcl-2/Bax mRNA in astrocytes and microvascular endothelial cells showed similar changes when cultured under hypoxic conditions, and treatment with iptakalim reversed these changes.
Fig. 5.
Effects of iptakalim (0 h after reperfusion) on the expression of caspase-3 protein in rat cortex after I/R injury
(a) Sham control group; (b) Rats that received MCAO; (c) Rats that received MCAO and iptakalim; (d) Results from the data analysis. Data are expressed as optical density optical (mean±SEM for four slides per group). * P<0.05 for rats that received MCAO compared with the sham control group; # P<0.05 for rats that received MCAO plus iptakalim compared with rats that received MCAO plus saline
Fig. 6.
Effects of iptakalim (0 h after reperfusion) on the expressions of Bcl-2 (a–c) and Bax (d–f) proteins in rat cortex after I/R injury
(a, d) Sham control group; (b, e) Rats that received MCAO; (c, f) Rats that received MCAO and iptakalim; (g) Results from the data analysis. Data are expressed as optical density optical (mean±SEM for four slides per group). * P<0.05 for rats that received MCAO compared with the sham control group; # P<0.05 for rats that received MCAO plus iptakalim compared with rats that received MCAO plus saline
Fig. 7.
Effects of iptakalim (Ipt; 0.01, 0.1, and 1 µmol/L) on the mRNA expression of caspase-3 (a) and the ratio of Bcl-2 mRNA expression to Bax mRNA expression (b) determined using real-time quantitative PCR in cultures of primary cerebrocortical neurons, astrocytes, and microvascular endothelial cells under hypoxic conditions
Hypoxic conditions were induced using 0.5 mmol/L Na2S2O4. Data are presented as the mean±SEM from four individual experiments. * P<0.05 for cells that received hypoxia condition compared with the normal control group; # P<0.05 for cells that were treated with iptakalim under hypoxia compared with cells exposed to hypoxia without iptakalim
4. Discussion
Our study demonstrates that administration of iptakalim coincident with or 1 h after reperfusion had significant neuroprotective effects against cerebral I/R injury: it decreased infarct volume, improved neurological function, ameliorated brain edema, and improved pathological changes in tissues and cells. Under hypoxic condition, iptakalim attenuated cell death, not only of neurons but also of astrocytes and endothelial cells. Iptakalim reduced LDH release in a concentration-dependent manner under hypoxic condition, and its protective effect was reversed by glibenclamide. The KATP channels-activating protective effect of iptakalim under hypoxic conditions was confirmed using NVU cells. We propose that the neuroprotective effects of iptakalim are complemented by concomitant neurovascular protection.
The fact that iptakalim administration coincident with or 1 h after reperfusion had significant neuroprotective effects against cerebral I/R injury suggests that iptakalim affects mechanisms that cause injury early in the ischemic process. As a KATP channel opener, iptakalim could activate KATP channels and, in conjunction with low energy status, reverse the intracellular calcium efflux, maintaining ion homeostasis. In ischemic brain, impaired cerebral blood flow caused a cerebral energy deficit and disturbed ion homeostasis. Several enzymatic systems can cause irreversible cell damages, which are also activated in ischemia. Potassium channel opener (KCO) may exert an effect early in ischemia by reducing the energy demands of the cell and thereby delaying activation of deleterious catabolic process. Iptakalim could also act by reducing the release of excitatory amino acids (Wang et al., 2004) and attenuating ischemic inhibition of protein kinases (Zhang et al., 2007). Cytotoxic edema also occurs early in the ischemic process. Iptakalim may help maintain tissue integrity and therefore reduce edema (Zhu et al., 2008). Although treatment with iptakalim at 2 h after reperfusion decreased infarct volume, it did not improve the neurological deficit or the extent of brain edema in rats, suggesting iptakalim may be involved in a delayed injury mechanism.
Overwhelming evidence suggests that both necrosis and apoptosis contribute significantly to cell death following I/R injury (Mattson et al., 2001). When brain cells suffer ischemia, oxidative stress, or other injury, the mitochondrial membrane potential decreases (Kowaltowski et al., 2001), and leads to the translocation of Bax from cytosol to mitochondria and release of cytochrome C (Kroemer, 2003). The Bcl-2 proteins can regulate this translocation (Green and Reed, 1998). A complex apoptosome can cause the formation of active caspase-3 (the apoptosis marker) (Li et al., 1995) and result in DNA fragmentation (Li et al., 1997). Our study showed that treatment with iptakalim after I/R injury significantly inhibited apoptosis in the rat cerebral cortex. Caspase-3 is a downstream molecular marker of apoptosis (Tsuchiya et al., 2003), and activation of caspase-3 leads to DNA fragmentation. Treatment with 8 mg/kg BW of iptakalim 1 h after reperfusion reduced caspase-3 mRNA and protein levels in rats that received MCAO. Our study also showed that treatment with iptakalim after reperfusion increased antiapoptotic Bcl-2 mRNA and protein expressions and decreased Bax mRNA and protein expressions. These results suggest that the inhibitory effects of iptakalim on apoptosis when administered after I/R injury may involve regulation of the balance between Bcl-2 (antiapoptosis) and Bax (pro-apoptosis) (Hu et al., 2004). Similar results were observed in vitro. Iptakalim inhibited caspase-3 mRNA expression and increased the ratio of Bcl-2 to Bax mRNA in NVU cells cultured under hypoxic conditions.
The mechanism responsible for the neuroprotective effects of iptakalim may involve protection of NVU cells from cell death. In our in vitro study, iptakalim protected NVU cells from hypoxia-induced cell necrosis and/or apoptosis. Iptakalim protected NVU cells from apoptosis by down-regulating the expression of caspase-3 and increasing the ratio of Bcl-2 to Bax in NVU cells. The protective effects of iptakalim on NVU cells subjected to hypoxic injury may involve inhibition of apoptosis.
Advances in central nervous system therapeutics and pathophysiological investigation have demonstrated the role of KATP channels in neuroprotection against cerebral injury caused by ischemia (Wang et al., 2004) and hypoxia-induced generalized seizure (Yamada et al., 2001; Seino and Miki, 2004). In addition to its expression in cerebral tissues (Liss et al., 1999), the SUR1 KATP channel is also expressed in pancreatic β cells. Diazoxide (Shimizu et al., 2002) is a commonly used KATP channel opener that interacts with SUR1 KATP channels. Diazoxide has side effects such as pathoglycemia. Nicorandil (Sato et al., 2000; Makins and Ballinger, 2003), another KATP channel opener, has severe gastrointestinal side effects. However, iptakalim, a fatty paramine structure, is more selective with SUR2 than with SUR1 and thus may have less severe side effects than diazoxide or nicorandil. As iptakalim has a high affinity for KATP channels in the brain (Wang et al., 2004), it would confer strong neuroprotection. Moreover, iptakalim is a small water-soluble compound and has little difficulty crossing the blood-brain barrier. Long-term systemic administration has proven that it has minimal toxic side effects (Wang et al., 2007).
Although KATP channels are involved in ischemic preconditioning, which has been used successfully in patients with a history of stroke, the potential for widespread application of ischemic preconditioning is limited. Ischemic preconditioning requires prior knowledge of the time of ischemic event. Although this is important for planning the ischemic preconditioning episodes, it is not possible to establish the time at which acute events will take place (Girn et al., 2007). The clinical use of cerebral ischemic preconditioning is only feasible when the occurrence of stroke can be predicted successfully. It is easier to predict the time of reperfusion than to predict the time of ischemia. Treatment after a stroke or an ischemic event may limit I/R-induced damage. In our study, we selected six time points for iptakalim treatment before or after cerebral I/R injury, and we examined the effects of iptakalim on cerebral injury 70 h after MCAO. Iptakalim had significant neuroprotective effects only when administered coincident with or 1 h after reperfusion. The neuroprotective effects of iptakalim combined with its biochemical properties, especially its SUR2 selectivity, suggest that iptakalim may prove to be useful as a clinical therapeutic agent for the treatment of stroke.
Our study demonstrates that post-event treatment with iptakalim had neuroprotective effects against cerebral I/R injury in a rat MCAO model. The neuroprotective effects of iptakalim appeared to be related to inhibition of apoptotic cell death of NVU cells. Further study is needed to determine whether iptakalim can be used clinically.
References
- 1.Abdel-Hamid KM, Baimbridge KG. The effects of artificial calcium buffers on calcium responses and glutamate-mediated excitotoxicity in cultured hippocampal neurons. Neuroscience. 1997;81(3):673–687. doi: 10.1016/S0306-4522(97)00162-0. [DOI] [PubMed] [Google Scholar]
- 2.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27(6):1124–1129. doi: 10.1161/01.STR.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 3.Chen YP, Qiu CR, Wang H. Cardiovascular pharmacological characterization of novel 2,3-dimethyl-2-butylamine derivatives in rats. Life Sci. 2004;75(17):2131–2142. doi: 10.1016/j.lfs.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Chen YP, Cui WY, Wang H. Selective actions of iptakalim on the subtypes of KATP channels. Chin Pharmacol Bull. 2006;22(3):278–284. [Google Scholar]
- 5.Diarra A, Sheldon C, Brett CL, Baimbridge KG, Church J. Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons. Neuroscience. 1999;93(3):1003–1016. doi: 10.1016/S0306-4522(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 6.Domoki F, Kis B, Nagy K, Farkas E, Busija DW, Bari F. Diazoxide preserves hypercapnia-induced arteriolar vasodilation after global cerebral ischemia in piglets. Am J Physiol. 2005;289(1):H368–H373. doi: 10.1152/ajpheart.00887.2004. [DOI] [PubMed] [Google Scholar]
- 7.Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 1998;814(1-2):41–54. doi: 10.1016/S0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- 8.Girn HR, Ahilathirunayagam S, Mavor AI, Homer-Vanniasinkam S. Reperfusion syndrome: cellular mechanisms of microvascular dysfunction and potential therapeutic strategies. Vasc Endovascular Surg. 2007;41(4):277–293. doi: 10.1177/1538574407304510. [DOI] [PubMed] [Google Scholar]
- 9.Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001;21(2):99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 11.Hamprecht B, Löffler F. Primary glial cultures as a model for studying hormone action. Methods Enzymol. 1985;109:341–345. doi: 10.1016/0076-6879(85)09097-8. [DOI] [PubMed] [Google Scholar]
- 12.Hu XL, Olsson T, Johansson IM, Brannstrom T, Wester P. Dynamic changes of the anti- and pro-apoptotic proteins Bcl-w, Bcl-2, and Bax with Smac/Diablo mitochondrial release after photothrombotic ring stroke in rats. Eur J Neurosci. 2004;20(5):1177–1188. doi: 10.1111/j.1460-9568.2004.03554.x. [DOI] [PubMed] [Google Scholar]
- 13.Janigro D, West GA, Gordon EL, Winn HR. ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. Am J Physiol. 1993;265(3 Pt 1):C812–C821. doi: 10.1152/ajpcell.1993.265.3.C812. [DOI] [PubMed] [Google Scholar]
- 14.Kis B, Kaiya H, Nishi R, Deli MA, Abraham CS, Yanagita T, Isse T, Gotoh S, Kobayashi H, Wada A, et al. Cerebral endothelial cells are a major source of adrenomedullin. J Neuroendocrinol. 2002;14(4):283–293. doi: 10.1046/j.1365-2826.2002.00778.x. [DOI] [PubMed] [Google Scholar]
- 15.Kis B, Snipes JA, Simandle SA, Busija DW. Acetaminophen-sensitive prostaglandin production in rat cerebral endothelial cells. Am J Physiol. 2005;288(4):R897–R902. doi: 10.1152/ajpregu.00613.2004. [DOI] [PubMed] [Google Scholar]
- 16.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495(1-2):12–15. doi: 10.1016/S0014-5793(01)02316-X. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G. Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun. 2003;304(3):433–435. doi: 10.1016/S0006-291X(03)00614-4. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome C and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Chopp M, Jiang N, Yao F, Zaloga C. Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1995;15(3):389–397. doi: 10.1038/jcbfm.1995.49. [DOI] [PubMed] [Google Scholar]
- 20.Linnik MD, Zobrist RH, Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke. 1993;24(12):2002–2008. doi: 10.1161/01.STR.24.12.2002. discussion 2008-2009. [DOI] [PubMed] [Google Scholar]
- 21.Liss B, Bruns R, Roeper J. Alternative sulfonylurea receptor expression defines metabolic sensitivity of KATP channels in dopaminergic midbrain neurons. EMBO J. 1999;18(4):833–846. doi: 10.1093/emboj/18.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009;40(3):S2–S3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makins R, Ballinger A. Gastrointestinal side effects of drugs. Expert Opin Drug Saf. 2003;2(4):421–429. doi: 10.1517/14740338.2.4.421. [DOI] [PubMed] [Google Scholar]
- 24.Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6(1-2):69–81. doi: 10.1023/A:1009676112184. [DOI] [PubMed] [Google Scholar]
- 25.Mederos y Schnitzler M, Derst C, Daut J, Preisig-Müller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J Physiol. 2000;525(2):307–317. doi: 10.1111/j.1469-7793.2000.t01-1-00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roof RL, Schielke GP, Ren X, Hall ED. A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke. 2001;32(11):2648–2657. doi: 10.1161/hs1101.097397. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Sasaki N, O′Rourke B, Marban E. Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels. J Am Coll Cardiol. 2000;35(2):514–518. doi: 10.1016/S0735-1097(99)00552-5. [DOI] [PubMed] [Google Scholar]
- 28.Seino S, Miki T. Gene targeting approach to clarification of ion channel function: studies of Kir6.x null mice. J Physiol. 2004;554(2):295–300. doi: 10.1113/jphysiol.2003.047175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu K, Lacza Z, Rajapakse N, Horiguchi T, Snipes J, Busija DW. MitoKATP opener, diazoxide, reduces neuronal damage after middle cerebral artery occlusion in the rat. Am J Physiol. 2002;283(3):H1005–H1011. doi: 10.1152/ajpheart.00054.2002. [DOI] [PubMed] [Google Scholar]
- 30.Simard JM, Chen M. Regulation by sulfanylurea receptor type 1 of a non-selective cation channel involved in cytotoxic edema of reactive astrocytes. J Neurosurg Anesthesiol. 2004;16(1):98–99. doi: 10.1097/00008506-200401000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Swanson RA, Sharp FR. Infarct measurement methodology. J Cereb Blood Flow Metab. 1994;14(4):697–698. doi: 10.1038/jcbfm.1994.88. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya D, Hong S, Matsumori Y, Shiina H, Kayama T, Swanson RA, Dillman WH, Liu J, Panter SS, Weinstein PR. Overexpression of rat heat shock protein 70 is associated with reduction of early mitochondrial cytochrome C release and subsequent DNA fragmentation after permanent focal ischemia. J Cereb Blood Flow Metab. 2003;23(6):718–727. doi: 10.1097/01.WCB.0000054756.97390.F7. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Zhang YL, Tang XC, Feng HS, Hu G. Targeting ischemic stroke with a novel opener of ATP-sensitive potassium channels in the brain. Mol Pharmacol. 2004;66(5):1160–1168. doi: 10.1124/mol.104.003178. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Tang Y, Wang L, Long CL, Zhang YL. ATP-sensitive potassium channel openers and 2,3-dimethyl-2-butylamine derivatives. Curr Med Chem. 2007;14(2):133–155. doi: 10.2174/092986707779313390. [DOI] [PubMed] [Google Scholar]
- 35.Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizu T, Seino S, Inagaki N. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292(5521):1543–1546. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- 36.Yanamoto H, Nagata I, Hashimoto N, Kikuchi H. Three-vessel occlusion using a micro-clip for the proximal left middle cerebral artery produces a reliable neocortical infarct in rats. Brain Res Brain Res Protoc. 1998;3(2):209–220. doi: 10.1016/S1385-299X(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 37.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25(8):1658–1664. doi: 10.1161/01.STR.25.8.1658. discussion 1664-1655. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. KATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol. 2004;37(4):857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Zhou F, Ding JH, Zhou XQ, Sun XL, Hu G. ATP-sensitive potassium channel opener iptakalim protects against MPP-induced astrocytic apoptosis via mitochondria and mitogen-activated protein kinase signal pathways. J Neurochem. 2007;103(2):569–579. doi: 10.1111/j.1471-4159.2007.04775.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhu HL, Luo WQ, Wang H. Iptakalim protects against hypoxic brain injury through multiple pathways associated with ATP-sensitive potassium channels. Neuroscience. 2008;157(4):884–894. doi: 10.1016/j.neuroscience.2008.09.033. [DOI] [PubMed] [Google Scholar]