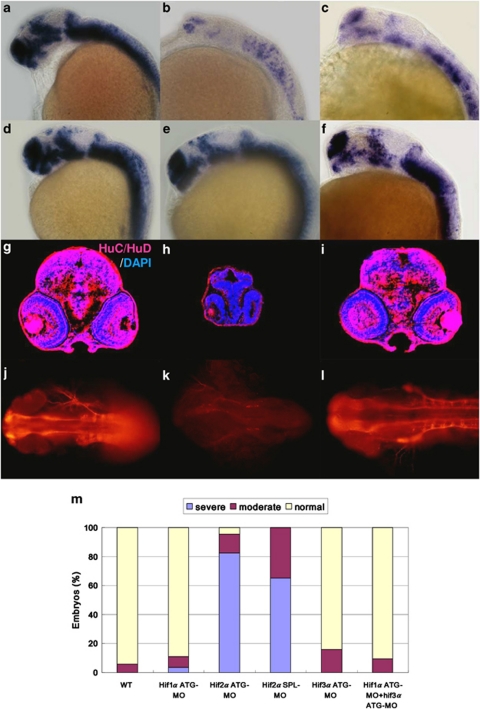
Figure 2.
Knockdown of hif2α impairs CNS development. (a–f) Lateral views of elavl3 expression in WT (a), hif2α ATG-MO (b), hif2α SPL-MO (c), hif1α ATG-MO (d), hif3α ATG-MO (e) and hif1α, hif3α dual ATG-MO (f) 24 h.p.f. embryos. elavl3 expression in the CNS was decreased greatly under the hif2α ATG-MO and hif2α SPL-MO treatments, indicating that these NPCs were not terminally differentiated. (g–i) Immunofluorescent staining of HuC/HuD (in red) in transverse brain sections of WT (g), hif2α ATG-MO (h) and hif1α, hif3α dual ATG-MO (i) 48 h.p.f. embryos. Nuclei are labeled by DAPI staining (in blue). Consistent with the mRNA transcriptional activity, knockdown of hif2α eliminated HuC expression, indicating a defect in neural differentiation. Knockdown of hif2α also caused morphological abnormality with small head. (j–l) Dorsal views of whole-mount immunostained images of acetylated α-tubulin in WT (j), hif2α ATG-MO (k) and hif1α/hif3α ATG-MO (l) 24 h.p.f. embryos. Axons were visualized by antibody staining for acetylated α-tubulin. The neural development defect following hif2α MO treatment was reflected by the loss of axon growth. (m) Percentages of normal, moderate and severe loss of elavl3 expression in the WT control (n=50), hif1α ATG-MO (n=54), hif2α ATG-MO (n=69), hif2α SPL-MO (n=23), hif3α ATG-MO (n=25) and (n=64) embryos. Knockdown of hif2α with either translation-blocking or splicing-blocking MOs led to a severe loss of elavl3 transcription, indicating impairment in neural differentiation