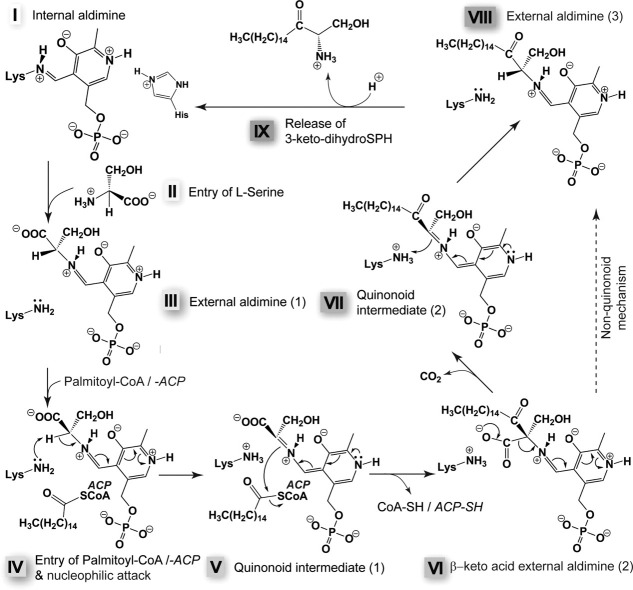
Figure 7.
Proposed SPT reaction mechanism.119,121 In the resting state, the PLP is linked to the enzyme via K265 (in SpSPT). Please note that the imidazole ring of H159 pi-stacks the pyridine ring of the PLP [see Fig. 6(B)]. For clarity, this residue is not shown in the subsequent steps (see comment below). The incoming amino acid (in this case serine) enters the active site (step II) and forms an external aldimine with PLP (step III). The external aldimine undergoes a nucleophilic attack, most likely by the internal aldimine-forming K265, after the second substrate, the acyl-CoA or the acyl-ACP (in this case palmitoyl), has entered the active site (step IV). The active site is expected to undergo conformational changes upon binding of the conjugated acyl. After the nucleophilic attack, a first quinonoid intermediate is formed (step V), the acyl carrier (either reduced CoA or ACP) leaves the active site, and a second external aldimine (with a β-keto acid) is formed (step VI). Decarboxylation of this intermediate produces the second quinonoid intermediate (step VII), which gets reprotonated to form the third external aldimine of the reaction cycle, between PLP and the reaction product (step VIII). The product 3-keto-dihydroSPH is then released (step IX). The proposed mechanism is based on the work of the Campopiano group.111,119,121 Importantly, H159 stabilizes by H-bond either the carboxyl group of the PLP-bound Ser (step III) or the carbonyl of the conjugated acyl (steps IV and V) and of the intermediates depicted in steps VI to VIII. For further explanations on the mechanism and especially the role of H159, please refer to the review by Ikushiro and Hayashi.106