Figure 10.
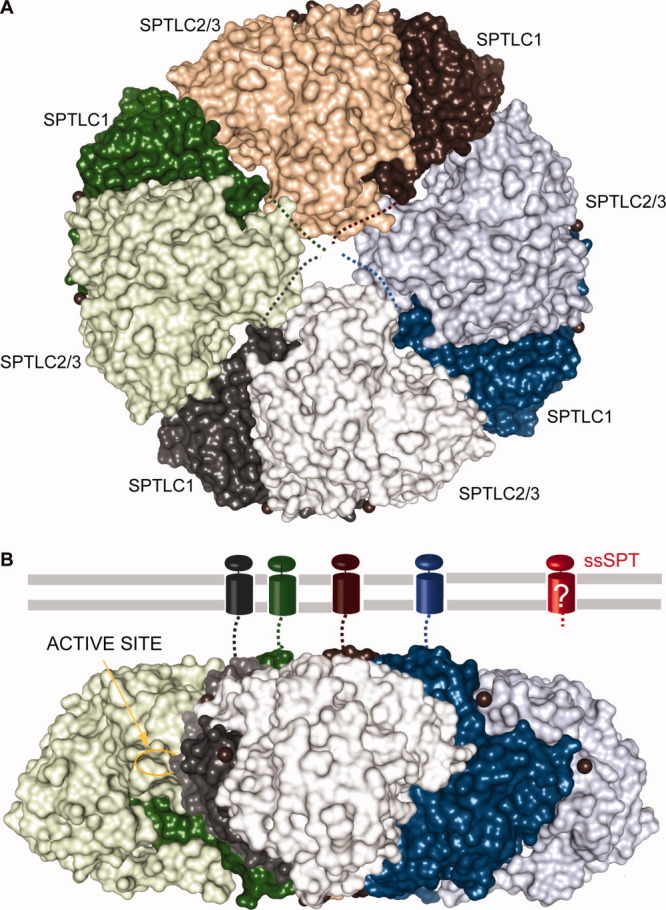
Putative membrane-bound SPT. Proposed topology of the membrane-bound mammalian and yeast SPT heteromer based on the assumption of Hornemann et al.140 SpSPT dimers in surface representation are colored green, brown, blue, and gray where SPTLC1 is colored with the darker shades and SPTLC2/3 with the darker shades. A: The orientation of the brown dimer corresponds to that of Figure 8(A), whereas the blue, gray, and green dimers are rotated by, respectively, 90, 180, and 270° in the plane of the sheet and assembled to form a disk. B: Lateral view of the octamer. Compared to the orientation in (A), the disk was rotated 90° anticlockwise along an axis parallel to the plane of the sheet. The four transmembrane helices of the SPTLC1 subunit are represented as in Figure 9. The active site of the green subunit SPTLC2/3 is indicated. Note that the topology of ssSPT, colored red, is unknown.
