Abstract
Purpose:
The objectives of this study were to compare the effects of caudal dexmedetomidine combined with ropivacaine to provide postoperative analgesia in children and also to establish its safety in the paediatric population.
Methods:
In a randomised, prospective, parallel group, double-blinded study, 60 children were recruited and allocated into two groups: Group RD (n=30) received 0.25% ropivacaine 1 ml/kg with dexmedetomidine 2 μg/kg, making the volume to 0.5 ml and Group R (n=30) received 0.25% ropivacaine 1 ml/kg + 0.5 ml normal saline. Induction of anaesthesia was achieved with 50% N2O and 8% sevoflurane in oxygen in spontaneous ventilation. An appropriate-sized LMA was then inserted and a caudal block performed in all patients. Behaviour during emergence was rated with a 4-point scale, sedation with Ramsay's sedation scale, and pain assessed with face, legs, activity, cry, consolability (FLACC) pain score.
Results:
The duration of postoperative analgesia recorded a median of 5.5 hours in Group R compared with 14.5 hours in Group RD, with a P value of <0.001. Group R patients achieved a statistically significant higher FLACC score compared with Group RD patients. The difference between the means of mean sedation score, emergence behaviour score, mean emergence time was statistically highly significant (P<0.001). The peri-operative haemodynamics were stable among both the groups.
Conclusion:
Caudal dexmedetomidine (2 μg/kg) with 0.25% ropivacaine (1 ml/kg) for paediatric lower abdominal surgeries achieved significant postoperative pain relief that resulted in a better quality of sleep and a prolonged duration of arousable sedation and produced less incidence of emergence agitation following sevoflurane anaesthesia.
Keywords: Analgesia, anaesthesia, caudal, dexmedetomidine, emergence agitation, postoperative period, ropivacaine, sevoflurane
INTRODUCTION
Pain is an unpleasant subjective sensation which can only be experienced and not expressed, especially in children, who rely completely on their parents or care-givers for their well-being. The concept of postoperative pain relief and its utilisation in the paediatric age group has improved dramatically over the recent years. Till date, various methods have evolved for providing post-op pain relief in paediatric population, nonetheless having some side effects which prohibit their use in children. For example, in children, narcotics could cause respiratory depression, oral analgesics cannot be given for some time after general anaesthesia due to the fear of vomiting and aspiration, and fear of needlestick in the case of parenteral analgesics.
The regional anaesthetic techniques significantly decrease the postoperative pain and systemic analgesic requirements. Caudal route is one of the simplest and safest techniques in paediatric surgery, with a high success rate. Caudal block is usually placed after the induction of general anaesthesia and is used as an adjunct to both intraoperative and postoperative analgesia in children undergoing surgical procedures below the level of the umbilicus. Caudal analgesia could reduce the amount of inhaled and intravenous (IV) anaesthetic administration, attenuate the stress response to surgery, facilitate a rapid, smooth recovery, and provide good immediate postoperative analgesia.[1] In order to decrease intra and postoperative analgesic requirements after single shot caudal epidural blockade, various additives, such as morphine, fentanyl, clonidine and ketamine, with local anaesthetics have been investigated.[2]
Ropivacaine, a long-acting amide local anaesthetic related structurally to bupivacaine, has been used for paediatric caudal anaesthesia. It provides pain relief with less motor blockade and is less cardiotoxic than bupivacaine, which makes it a more suitable agent for caudal epidural analgesia, especially following day care surgery.[3] Dexmedetomidine is an α2 agonist having an eightfold greater affinity for α2 adrenergic receptors than clonidine and much less α1 effects. A major advantage of dexmedetomidine is its higher selectivity compared with clonidine for α2A receptors which is responsible for the hypnotic and analgesic effects.[4]
The objectives of this study were to compare the effects of dexmedetomidine combined with ropivacaine to provide postoperative analgesia and also determine the other effects of dexmedetomidine when added to ropivacaine for caudal analgesia in children undergoing lower abdominal surgeries.
METHODS
Study design
This was a randomised, prospective, parallel group, double-blinded study.
Randomisation
Simple randomised sampling was done by lottery method.
Sample size
Sixty patients were studied.
Inclusion criteria
ASA I/II patients between 6 months and 6 years of age undergoing lower abdominal surgeries were included.
Exclusion criteria
Patients with known allergy to the study drugs, suspected coagulopathy, infection at the site of caudal block, history of developmental delay, neurological diseases and skeletal deformities were excluded.
Allocation
After obtaining institutional ethical committee and review board's approval and written informed consent from the parents, the children were randomly allocated into two groups. Group R (n=30) was taken as ropivacaine group and Group RD (n=30) as dexmedetomidine group.
Intervention
Caudal administration of drug mixture
Group RD (n=30) – 0.25% ropivacaine 1 ml/ kg with dexmedetomidine 2 μg/kg, making the volume to 0.5 ml
Group R (n=30) – Caudal 0.25% ropivacaine 1 ml/kg + 0.5 ml normal saline
Masking
Anaesthesiologist who administered the drug and the observer were blinded to the study. Sterile syringes containing equal volumes of drug or placebo were loaded by another anaesthesiologist not concerned or participating in the study. The intraoperative monitoring and postoperative observation was done by the same anaesthesiologist who administered the drug or placebo, but was unaware of the content of the syringes.
Pre-op evaluation
In all children, age, body weight, and baseline vital parameters were recorded. History regarding previous anaesthesia, surgery, any significant medical illness, medications and allergy was recorded. Complete physical examination and airway assessment were done. The following laboratory investigations were done: Haemoglobin percentage, blood sugar, urea, serum creatinine and urine analysis.
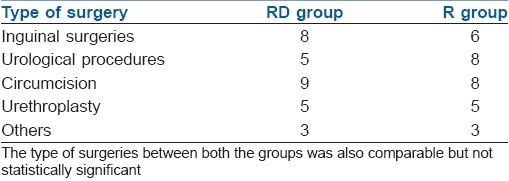
All the patients were pre-medicated with oral midazolam 0.5 mg/kg, 1 hour prior to induction. Induction of anaesthesia was achieved with 50% N2O and 8% sevoflurane in oxygen in spontaneous ventilation. An appropriate-sized laryngeal mask airway (LMA) was inserted. After the insertion of LMA, sevoflurane concentration was reduced to 3% in 50% nitrous oxide, patients were left in spontaneous ventilation and a caudal block was performed in all patients according to the group. The inhaled concentration of sevoflurane was adjusted to achieve haemodynamic changes less than 30% of the baseline values.[4] No other narcotics, analgesics or sedatives were used intraoperatively. The types of the various surgical procedures done are summarised in Table 1. Standard monitoring was used during anaesthesia and surgery. Heart rate (HR), mean arterial pressure (MAP) and oxygen saturation (SpO2) were recorded before surgery and every 5 min till the end of surgery. The occurrence of intraoperative hypotension requiring a fluid bolus, bradycardia requiring atropine, and the maximum maintenance concentration of sevoflurane (%) were recorded.
Table 1.
Comparison of type of surgery in both groups
Behaviour during emergence was rated on a 4-point scale:[5,6]
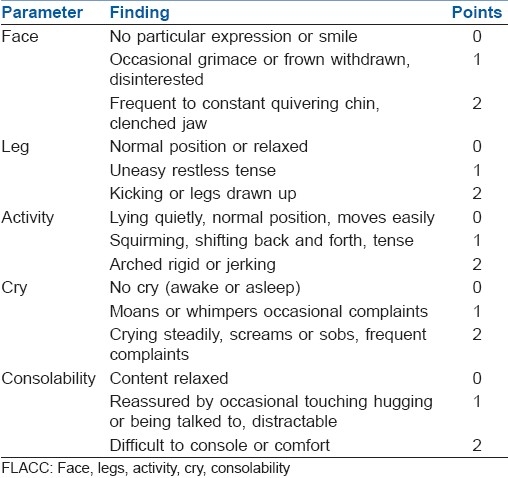
1) calm; 2) not calm but could be easily calmed; 3) not easily calmed, moderately agitated or restless; and 4) combative, excited, or disoriented. Using the paediatric observational face, legs, activity, cry, consolability (FLACC) pain score[7] [Table 2] with its 0–10 score range, each patient's pain intensity was assessed at the end of surgery and then every 4 hours for 24 hours after operation. If the FLACC pain score was 4 or more, syrup paracetamol 15 mg/kg was administered. The duration of analgesia (from the time of caudal injection to the time at which FLACC score was 4 or more) was also recorded.
Table 2.
FLACC score
Sedation score was assessed using Ramsay's sedation scale[8] as follows:
anxious and agitated or restless, or both
co-operative, oriented, and calm
responsive to commands only
exhibiting brisk response to light glabellar tap or loud auditory stimulus
exhibiting a sluggish response to light glabellar tap or loud auditory stimulus
unresponsive
The following times were recorded:
The anaesthesia time (time from induction of anaesthesia to the end of surgery when sevoflurane was discontinued).
Time from caudal block to skin incision.
Time from caudal block to end of surgery.
Emergence time (time from the end of surgery to opening the eyes on calling).
Complications such as postoperative nausea and vomiting (PONV), respiratory depression, urinary retention, hypotension and bradycardia were also noted. Respiratory depression was defined as a decrease in SpO2 of of less than 95% requiring supplementary oxygen. Hypotension was defined as systolic arterial pressure 70 plus twice the age in years and associated with altered peripheral perfusion. Bradycardia was defined as HR below 80 beats/min for age 1 year and 60 beats/min for ages above 1 year. Delayed anaesthetic emergence was defined as 20 min elapsing from the end of surgery to exiting the operating theatre.
Failure of caudal block was defined as any increase in HR or MAP more than 20% of the pre-incision values. In our study, we encountered eight failed caudal blocks. Those cases were eliminated from the study.
Statistical analysis
Data were analysed using SPSS version 13.0 computer software. Numerical variables were presented as mean and standard deviation (SD) and categorical variables were presented as frequency (%). Student's t test was used for between-group comparisons between categorical variables. Time to first analgesic administration was analysed by the Kaplan-Meier survival analysis.[4] A P value of <0.05 was taken to be significant and a P value of <0.001 was considered highly significant.
The pilot study sample statistics revealed that the required sample size in each group was 13 subsets to detect a difference in the average time to first analgesic time as small as 3.35 times. The standard deviations of the two groups were 3.6 and 1.1 hours of RD and R groups, respectively. The level of significance and the power of the study were fixed as 0.05 (α) and n0.9 (1 – β). The sample size was increased by more than two fold to avoid the skewness of the primary outcome variable (time to first analgesics) with the possibility of existence of censored data. Now the sample size of the study was 30 subsets in each group.
The power of the univariate general linear model test was found to be unity for detecting the difference between the mean survival time 14.4 and 5.5 hours during the observation period of 24 hours at α=0.05.
RESULTS
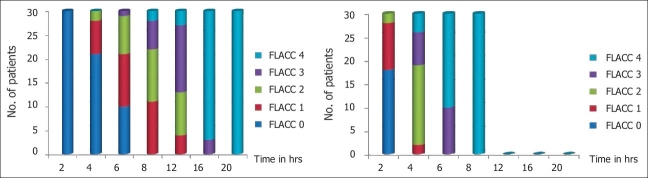
The duration of postoperative analgesia [Figure 1] recorded a median of 5.5 hours and 95% confidence interval (CI) of (4.97–6.03) in Group R compared with 14.5 hours (13.90–15.09) in Group RD, with a P value of <0.001. There was a significant difference between the groups in the FLACC score [Figure 2] measured 4th hourly in the postoperative period. Group R patients achieved significantly higher FLACC score compared with Group RD patients, where 20 out of 30 children achieved a FLACC score of 4 at 6th hour compared with 0 patients in Group RD, whereas Group RD children had FLACC score of 4 at 16th hour of postoperative period. The difference of mean sedation score between both the groups was statistically very highly significant (P<0.001). RD group had significant sedation compared to R group which means that RD group children were asleep but easily arousable. The emergence behaviour score [Table 3] of the RD group was 1.3±0.4 and that of the R group was 3.4±0.5. The difference between the means was statistically highly significant (P<0.001). This means that R group children were agitated and restless compared to RD group children who were calm and co-operative. The mean emergence time [Table 3] of RD group was 5.4±1.8 min and that of the R group was 4.0±1.0 min. The difference of mean between the two groups was statistically very highly significant (P<0.001). The pre-op, intra-op and post-op haemodynamic changes between the groups [Figure 3] were comparable and were not statistically significant and therapeutic interventions were not required.
Figure 1.
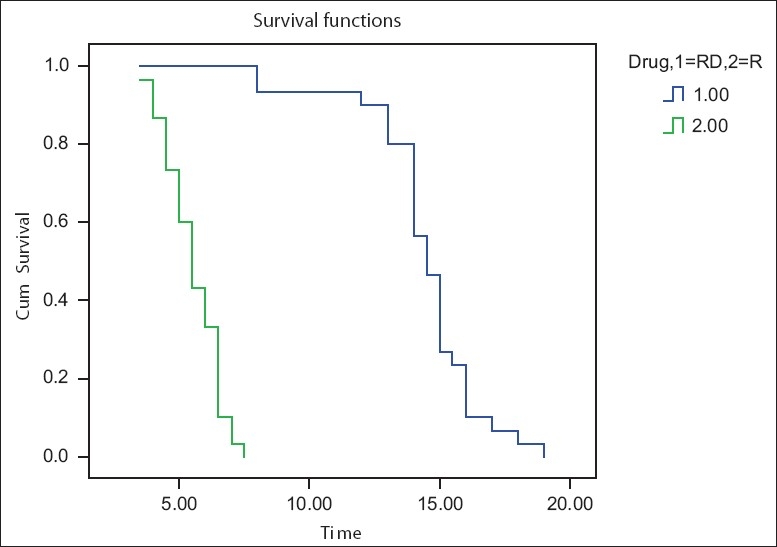
Comparison of duration of analgesia (Kaplan-Meier survival curve)
Figure 2.
FLACC score of and RD and R group children. There was a significant difference between the groups in the FLACC score measured 4th hourly in the postoperative period. Group R patients achieved significantly higher FLACC score compared with Group RD children. Twenty out of 30 children achieved a FLACC score of 4 at 6th hour in Group R compared with 0 patients in Group RD, whereas in Group RD, the children had FLACC score 4 at 16th hour of the postoperative period
Table 3.
Comparison of emergence time and emergence behaviour score
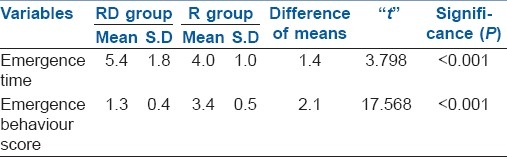
Figure 3.
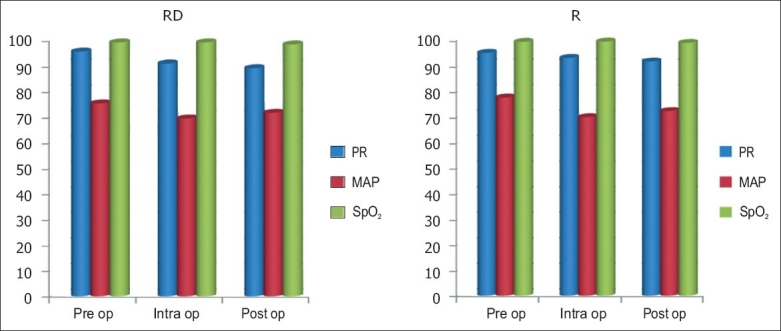
Comparison of haemodynamic variables. The pre-op, intra-op and post-op haemodynamic changes between the groups were comparable and were not statistically significant and therapeutic interventions were not required
DISCUSSION
Ropivacaine, in comparison to bupivacaine, has a wider margin of safety, less motor blockade, less cardiovascular or neurological toxicity and similar duration of analgesia. It can be safely used for regional anaesthesia and analgesia in the ambulatory setting in paediatrics.[1–3,7,9,10]
Like clonidine,[11,12] dexmedetomidine also enhances the effects of local anaesthetics without increasing the incidence of side effects.[13] Dexmedetomidine compared to clonidine is a much more selective α2-adrenoceptor agonist, which might permit its application in relatively high doses for sedation and analgesia without the unwanted vascular effects from activation of α1-receptors. In addition, dexmedetomidine is a shorter-acting drug than clonidine and it is unique that its sedative effect can be reversed by atipamezole. These properties render dexmedetomidine suitable for sedation and analgesia during the whole perioperative period. The preferred route of administration of dexmedetomide is the intravenous (IV) route, although others have been studied. In children, the pharmacokinetics of a 10-min IV infusion of dexmedetomidine, 0.33, 0.66, or 1 μg/ kg, yielded a rapid redistribution (α phase) half-life of 9 min and a slow (β phase) elimination phase with a half-life of 2 hours, similar to adults.[14] There appears to be no dose-dependent kinetics in children. Pharmacodynamic effects of dexmedetomidine have been studied thoroughly in adults, whereas in children, initial publications were anecdotal, in the form of case reports.[15–18] More recently, investigations in children have described the pharmacokinetics as well as a number of pharmacodynamic effects in randomised, controlled trials.[19–21] One of the major advantages of dexmedetomidine over other sedatives is its respiratory effects, which are minimal in adults and children. Indeed, respiratory rate, carbon dioxide (CO2) tension, and oxygen saturation are generally maintained during dexmedetomidine sedation in children. Dexmedetomidine provides an interesting quality of sedation that permits arousal with gentle stimulation. It has been studied for sedation in children for a number of different purposes, including radiologic procedures such as magnetic resonance imaging (MRI).[19,20]
El-Hennawy et al.[4] administered dexmedetomidine and clonidine, both in a dose of 2 μg/kg as adjuvant with 0.25% bupivacaine caudally. They found that the duration of analgesia was significantly higher in the group receiving bupivacaine–dexmedetomidine mixture [median (95% CI): 16 hours (14–18)] or bupivacaine–clonidine mixture [median (95% CI): 12 hours (3–21)] than the group receiving bupivacaine alone [median (95% CI): 5 hours (4–6)]. Neogi et al.[22] compared clonidine 1 μg/kg and dexmedetomidine 1 μ/kg as adjuncts to ropivacaine 0.25% for caudal analgesia in paediatric patients and concluded that addition of both clonidine and dexmedetomidine with ropivacaine administered caudally significantly increases the duration of analgesia. The patients stayed haemodynamically stable and there were no undue side effects. The mean duration of analgesia was 6.32±0.46 hours in ropivacaine group, 13.17±0.68 hours in clonidine group and 15.26±0.86 hours in dexmedetomidine group. The prolongation of duration of analgesia was significant in both clonidine and dexmedetomidine groups when compared to ropivacaine alone administered group. The incidence of adverse effects was statistically insignificant between the three groups.
We observed from our study that the duration of postoperative analgesia [Figure 1] recorded a median of 14.5 hours (13.90–15.09) in Group RD compared with 5.5 hours (4.97–6.03) in Group R, with a P-value of <0.001. Group R patients achieved a statistically significant higher FLACC score compared with Group RD patients [Figure 2]. The pre-op, intra-op and post-op haemodynamic variables [Figure 3] between the groups were comparable and were not statistically significant and therapeutic interventions were not required. No episodes of clinically significant postoperative complications such as PONV, respiratory depression, urinary retention, pruritus, hypotension and bradycardia were observed. The results of our observations show that in addition to prolonged post-op analgesia, dexmedetomidine has a favourable safety profile and stable haemodynamics, which are in concordance with the reports published by several other authors.[4,5,8,12–17]
Emergence agitation (EA)[23–28] is a frequent side effect of sevoflurane anaesthesia in paediatric patients. There is no well-defined prophylaxis or treatment, although the incidence of this excitatory behaviour seems to be reduced by the perioperative use of sedative and analgesic drugs. The α2 receptor agonists may offer advantages in preventing EA because they have both analgesic and sedative properties.
Saadawy et al.[29] compared caudal bupivacaine 0.25% administered with dexmedetomidine 1 μg/kg and caudal bupivacaine alone and showed that the incidence of agitation following sevoflurane anaesthesia was significantly lower with dexmedetomidine (P<0.05). The duration of analgesia was significantly longer with dexmedetomidine administration (P<0.001). No statistically significant difference in haemodynamics was found between both the groups. Dexmedetomidine produced better quality of sleep and a prolonged duration of sedation (P<0.05).
Bock et al.[30] studied the effect of clonidine on EA in 80 children aged 3–8 years undergoing minor day-case surgery who were anaesthetised with sevoflurane. The children received a caudal block for perioperative pain relief. A dose of 3 μg/kg clonidine was found to prevent agitation, whether administered IV or caudally.
Using caudal dexmedetomidine 2 μg/kg with sevoflurane anaesthesia, we found the mean emergence time [Table 3] of RD group was 5.4±1.8 min and the same in the R group was 4.0±1.0 min. The difference of mean between the two groups was statistically very highly significant (P<0.001). The emergence behaviour score [Table 3] of the RD group was 1.3±0.4 and that of the R group was 3.4±0.5. The difference between the means was statistically highly significant (P<0.001). The observations showed that R group children were agitated and restless compared to RD group children who were calm and co-operative. This implies that caudally administered dexmedetomidine prevented the EA following sevoflurane administration significantly. The difference between mean sedation scores of both the groups was statistically very highly significant (P<0.001). RD group had significant sedation compared to R group, meaning that RD group children were asleep but easily arousable.
CONCLUSION
Caudal dexmedetomidine 2 μg/kg with 0.25% ropivacaine 1 ml/kg for paediatric lower abdominal surgeries achieved significant postoperative pain relief up to 15 hours, which resulted in a better quality of sleep and a prolonged duration of arousable sedation and produced less incidence of EA following sevoflurane anaesthesia. Also, there was no necessity for any supplemental analgesic and the haemodynamics too were stable. No episodes of clinically significant postoperative complications were observed. Hence, we find dexmedetomidine to be a safe and effective adjuvant for caudal analgesia in paediatrics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Deng XM, Xiao WJ, Tang GZ, Luo MP, Xu KL. The minimum local anesthetic concentration of ropivacaine for caudal analgesia in children. Anesth Analg. 2002;94:1465–8. doi: 10.1097/00000539-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Birbicer H, Doruk N, Cinel I, Atici S, Avlan D, Bilgin E, et al. Could adding magnesium as adjuvant to ropivacaine in caudal anaesthesia improve postoperative pain control. Pediatr Surg Int. 2007;23:195–8. doi: 10.1007/s00383-006-1779-4. [DOI] [PubMed] [Google Scholar]
- 3.Ray M, Mondal SK, Biswas A. Caudal analgesia in paediatric patients: Comparison between Bupivacaine and Ropivacaine. Indian J Anaesth. 2003;47:275–8. [Google Scholar]
- 4.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 5.Ibacache M, Muñoz H, Brandes V. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia. Anesth Analg. 2004;98:60–3. doi: 10.1213/01.ANE.0000094947.20838.8E. [DOI] [PubMed] [Google Scholar]
- 6.Aono J, Ueda W, Mamiya K. Greater incidence of delirium during recovery from sevoflurane in preschool boys. Anesthesiology. 1997;87:1298–300. doi: 10.1097/00000542-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Merkel SI, Voeoel-Lewus T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 8.Ramsay MA, Kuterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 9.De Negri P, Ivani G, Visconti C, de Vivo P. How to prolong postoperative analgesia after caudal anaesthesia with ropivacaine in children: S-ketamine versus clonidine. Pediatr Anaesth. 2001;11:679–83. doi: 10.1046/j.1460-9592.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- 10.Ivani G, De Negri P, Lonnqvist PA, L’Erario M, Mossetti V, Difilippo A, et al. Caudal anesthesia for minor pediatric surgery: A prospective randomized comparison of ropivacaine 0.2% vs levobupivacaine 0.2% Paediatr Anaesth. 2005;15:491–4. doi: 10.1111/j.1460-9592.2004.01536.x. [DOI] [PubMed] [Google Scholar]
- 11.Eisenach JC, De Kock M, Klimscha W. Alpha sub 2 -Adrenergic Agonists for Regional Anesthesia: A Clinical Review of Clonidine (1984 - 1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Hansen TG, Henneberg SW, Walther-Larsen S, Lund J, Hansen M. Caudal bupivacaine supplemented with caudal or intravenous clonidine in children undergoing hypospadias repair: A doubleblind study. Br J Anaesth. 2004;92:223–7. doi: 10.1093/bja/aeh028. [DOI] [PubMed] [Google Scholar]
- 13.Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an á2A Adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 14.Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006;105:1098–110. doi: 10.1097/00000542-200612000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Tobias JD, Berkenbosch JW. Initial experience with dexmedetomidine in paediatric-aged patients. Paediatr Anaesth. 2002;12:171–5. doi: 10.1046/j.1460-9592.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 16.Ard J, Doyle W, Bekker A. Awake craniotomy with dexmedetomidine in pediatric patients. J Neurosurg Anesthesiol. 2003;15:263–6. doi: 10.1097/00008506-200307000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Berkenbosch JW, Wankum PC, Tobias JD. Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children. Pediatr Crit Care Med. 2005;6:435–39. doi: 10.1097/01.PCC.0000163680.50087.93. [DOI] [PubMed] [Google Scholar]
- 18.Nichols DP, Berkenbosch JW, Tobias JD. Rescue sedation with dexmedetomidine for diagnostic imaging: A preliminary report. Paediatr Anaesth. 2005;15:199–203. doi: 10.1111/j.1460-9592.2005.01416.x. [DOI] [PubMed] [Google Scholar]
- 19.Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103:63–7. doi: 10.1213/01.ANE.0000219592.82598.AA. [DOI] [PubMed] [Google Scholar]
- 20.Koroglu A, Demirbilek S, Teksan H, Sagir O, But AK, Ersoy MO. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: Preliminary results. Br J Anaesth. 2005;94:821–4. doi: 10.1093/bja/aei119. [DOI] [PubMed] [Google Scholar]
- 21.Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006;105:1098–110. doi: 10.1097/00000542-200612000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Neogi M, Bhattacharjee DP, Dawn S, Chatterjee N. A comparative study between clonidine and dexmedetomidine used as adjuncts to ropivacaine for caudal analgesia in paediatric patients. J Anaesthesiol Clin Pharmacol. 2010;26:149–53. [Google Scholar]
- 23.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: Many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 24.Jöhr M. Excitation following sevoflurane: A problem in pediatric anesthesia? Anaesthesist. 1999;48:917–8. doi: 10.1007/s001010050807. [DOI] [PubMed] [Google Scholar]
- 25.Cole JW, Murray DJ, McAllister JD, Hirshberg GE. Emergence behaviour in children: Defining the incidence of excitement and agitation following anaesthesia. Paediatr Anaesth. 2002;12:442–7. doi: 10.1046/j.1460-9592.2002.00868.x. [DOI] [PubMed] [Google Scholar]
- 26.Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Pediatr Anesth. 2005;15:1098–104. doi: 10.1111/j.1460-9592.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- 27.Weldon BC, Bell M, Craddock T. The effect of caudal analgesia on emergence agitation in children after sevoflurane versus halothane anesthesia. Anesth Analg. 2004;98:321–6. doi: 10.1213/01.ANE.0000096004.96603.08. [DOI] [PubMed] [Google Scholar]
- 28.Tobias JD, Berkenbosch JW, Russo P. Additional experience with dexmedetomidine in pediatric patients. South Med J. 2003;96:871–5. doi: 10.1097/01.SMJ.0000053557.75799.09. [DOI] [PubMed] [Google Scholar]
- 29.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–6. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 30.Bock M, Kunz P, Schreckenberger R, Graf BM, Martin E, Motsch J. Comparison of caudal and intravenous clonidine in the prevention of agitation after sevoflurane in children. Br J Anaesth. 2002;88:790–6. doi: 10.1093/bja/88.6.790. [DOI] [PubMed] [Google Scholar]