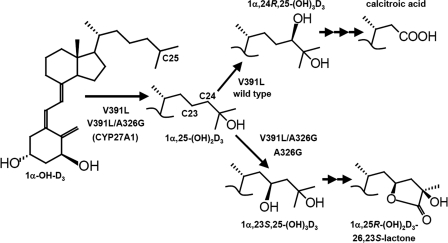
FIGURE 5.
Summary of observed metabolism by wild-type CYP24A1 and its V391L mutants. CYP24A1 catalyzes the catabolism of 1α,25-(OH)2D3 and 25-OH-D3 via C24 or C23 lactone pathways but does not significantly metabolize the prodrug, 1α-OH-D3. The mutation V391L broadened the substrate specificity to accommodate the anabolic activation of 1α-OH-D3 to produce 1α,25-(OH)2D3, which was subsequently catabolized by the C24 pathway. When combined with the A326G mutation, previously shown to direct catabolism toward the C23 lactone, the double mutant converted 1α-OH-D3 to 1α,25-(OH)2D3 and catabolized it via the C23 lactone pathway. Overall, 1α-hydroxylated prodrugs, without sterically hindering modifications on the side chain, appeared most conducive to anabolic C25-hydroxylation by V391L and, to a lesser extent, vitamin D3, which lacks a 1α-hydroxyl (not shown; supplemental Figs. S5). However, analogs investigated possessing a C25-hydroxyl group were easily catabolized by V391L whether formed in situ or given directly as substrate.