FIGURE 3.
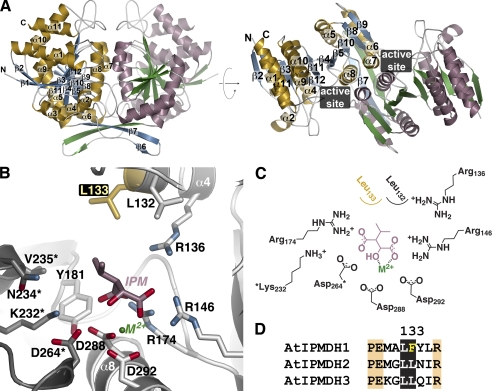
Structure of AtIPMDH2. A, ribbon diagrams of the AtIPMDH2 dimer. Monomer A is shown with gold α-helices and blue β-strands and monomer B is drawn with rose α-helices and green β-strands. Secondary structure features are labeled on the A monomer. The right view is rotated 90° to show the two domains of each monomer. The position of the active site cleft is indicated. B, active site view and model of 3-isopropylmalate (IPM) and divalent metal (M2+). Side chains of active site residues are shown with those from the adjacent subunit (gray) indicated by an asterisk. The positions of the substrate and metal are modeled based on the bacterial structures (20, 24, 25). The active site difference among the AtIPMDH isoforms is highlighted in gold. C, schematic of the active site model. D, sequence comparison of the region including residue 133 (AtIPMDH2 numbering).