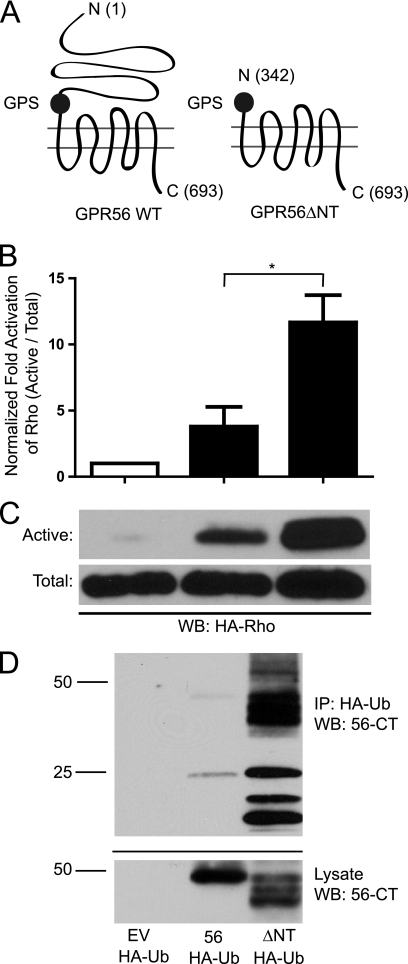
FIGURE 2.
N-terminal truncation enhances GPR56-mediated signaling and receptor ubiquitination. A, schematic diagram showing the location of the N-terminal GPR56 truncation. The numbers in parentheses indicate the amino acid positions of the N terminus, the GPS domain starting position, and the C terminus for each construct. B, quantification of active RhoA via pull-down with GST-RBD. Total Rho was first normalized between samples before probing for active Rho. Active Rho levels were compared with empty vector-transfected cells. *, p < 0.05; n = 10. WB, Western blotting. C, top panel, Western blot analysis of active RhoA pull-down with GST-RBD beads from HEK-293 cells transfected with empty vector (EV), wild-type GPR56, or the ΔNT mutant. Bottom panel, Western blot analysis of total RhoA levels from HEK-293 cells transfected with empty vector, GPR56, and GPR56ΔNT. D, ubiquitination of wild-type versus truncated GPR56. Full-length GPR56 or GPR56ΔNT were transfected into HEK-293 cells with HA-Ubiquitin (HA-Ub). Immunoprecipitation (IP) was performed with anti-HA antibodies and immunoprecipitates were probed via Western blot analysis with anti-GPR56-CT antibodies to visualize ubiquitinated GPR56. The data shown are representative of three independent experiments.