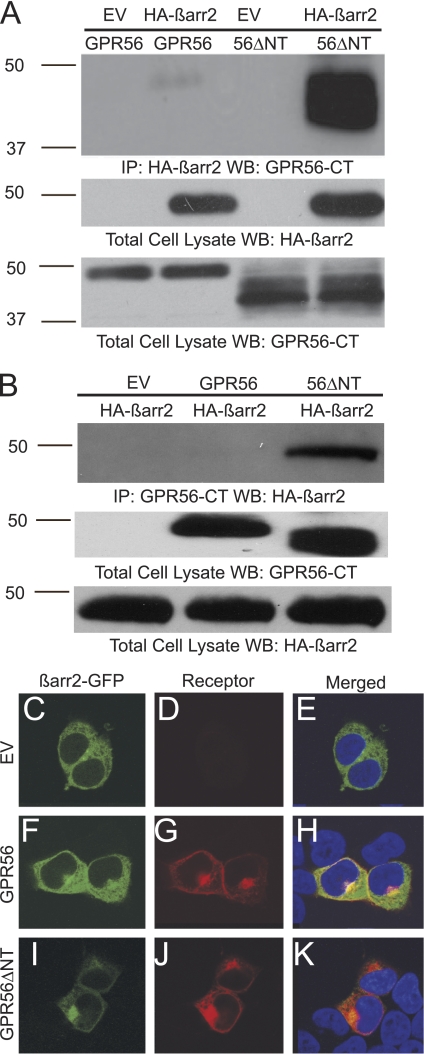
FIGURE 3.
β-arrestin 2 binds avidly to GPR56ΔNT and attenuates ΔNT-induced cytotoxicity. A, HEK-293 cells were transfected with GPR56 or GPR56ΔNT in the absence or presence of HA-β-arrestin 2 (HA-β-arr2). Immunoprecipitation (IP) was performed with HA antibody coupled to agarose beads. Coimmunoprecipitation of GPR56 was detected by Western blotting (WB) with the anti-GPR56-CT antibody. EV, empty vector. B, HEK-293 cells were transfected with HA-β-arrestin 2 and either empty vector, GPR56, or GPR56ΔNT. Immunoprecipitation was performed with the anti-GPR56-CT antibody and protein A/G agarose. Coimmunoprecipitation of β-arrestin 2 was detected by Western blotting with anti-HA antibody. C–K, wild-type GPR56 and GPR56ΔNT promote β-arrestin 2 cellular redistribution and perinuclear aggregation. β-Arrestin 2-GFP expressed alone was distributed evenly throughout HEK-293 cells (C and E), but co-expression with GPR56 promoted translocation of β-arrestin 2 to the perinuclear region where it colocalized with the receptor (H). Translocation of β-arrestin 2 to the perinuclear region was even more dramatic upon coexpression with GPR56ΔNT (K). DAPI staining is shown in E, H, and K. These data are representative of four independent experiments.