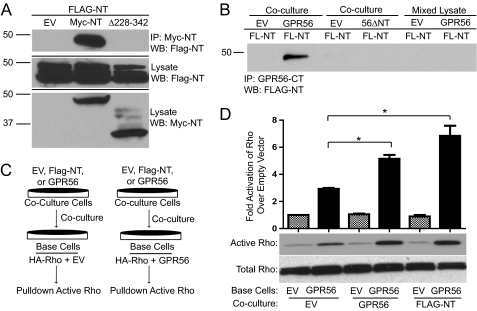
FIGURE 5.
GPR56 NT-NT interactions enhance GPR56-mediated signaling. A, coimmunoprecipitation (IP) between FLAG-GPR56-NT and Myc-GPR56-NT. HEK-293 cells were separately transfected with FLAG-NT, empty vector (EV), Myc-NT, or Myc-NTΔ228–342. After 24 h, FLAG-NT transfected cells were cocultured with empty vector- (first lane), Myc-NT- (second lane), or Myc-NTΔ228–342 (third lane)-transfected cells. The next day, cells were lysed, and immunoprecipitation was performed with protein A/G beads coupled to c-myc antibody, followed by Western blotting (WB) for FLAG-NT. B, HEK-293 cells transfected with FLAG-NT (FL-NT) were cocultured with HEK-293 cells transfected with empty vector (first and third lanes), GPR56 wild-type (second lane), or GPR56ΔNT mutant (fourth lane). After coculturing for 24 h, immunoprecipitation was performed with protein A/G beads coupled to GPR56-CT antibody, followed by Western blotting for FLAG-NT. In parallel experiments, FLAG-NT-expressing cells were grown separately, harvested, and then mixed with lysates from cells transfected with empty vector (fifth lane) or GPR56 wild-type (sixth lane) prior to immunoprecipitation with anti-GPR56-CT antibodies. The data shown are representative of three to five independent experiments for the various conditions. C, schematic drawing of coculturing technique. HEK-293 cells were transfected with HA-RhoA plus empty vector or GPR56 wild-type. These base cells were then cocultured for 24 h with cells expressing EV, GPR56, or FLAG-NT. The activity of HA-Rho in the base cells was then measured and expressed as fold over EV/EV cells. D, quantification of active Rho in coculturing experiments. Also included is the Western blot analysis of active and total Rho. A significant difference in GPR56-mediated RhoA activation was seen for base cells cocultured with cells expressing GPR56 (*, p < 0.05; n = 3) and FLAG-NT (*, p < 0.04; n = 3).