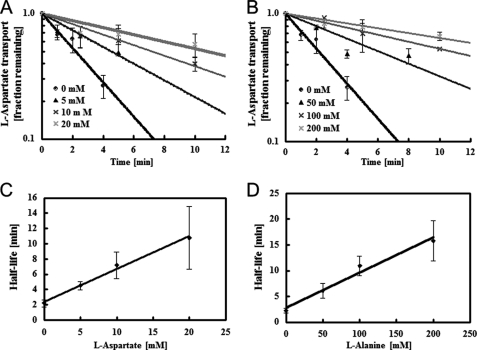
FIGURE 4.
Substrate protection of solubilized AspT. A, solubilized AspT (27 μg of protein/ml) was incubated at 37 °C in the absence (●) or presence of aspartate at 5 (▴), 10 (×), or 20 mm (*). To follow the decay of AspT, aliquots were removed at the indicated times and placed in chilled quench tubes containing 20 mm l-aspartate and the other components required for reconstitution. After reconstitution, proteoliposomes were tested for residual AspT activity by use of an abbreviated assay (see “Experimental Procedures”). In the absence of added substrate, recoverable AspT activity disappeared with a half-life of 0.15 min. Aspartate concentrations of 5, 10, and 20 mm gave half-lives of 4.5, 7.2, and 11 min, respectively; from Equation 1 (see text), the predicted corresponding KD(l-aspartate) value was 4.6 ± 0.4 mm. B, solubilized AspT was incubated in the absence (●) or presence of l-alanine (50 mm, ▴; 100 mm, ×; 200 mm, *) and residual AspT activity was measured by use of an abbreviated assay. In the absence of added substrate, recoverable AspT activity disappeared with a half-life of 0.15 min. Alanine concentrations of 50, 100, and 200 mm gave half-lives of 6.1, 11, and 16 min, respectively; from Equation 1, the predicted corresponding KD(l-alanine) value was 29 ± 4 mm. C and D, half-life of solubilized AspT in the presence of l-aspartate (C) and l-alanine (D). Data are from three independent experiments and presented as the average with S.D.