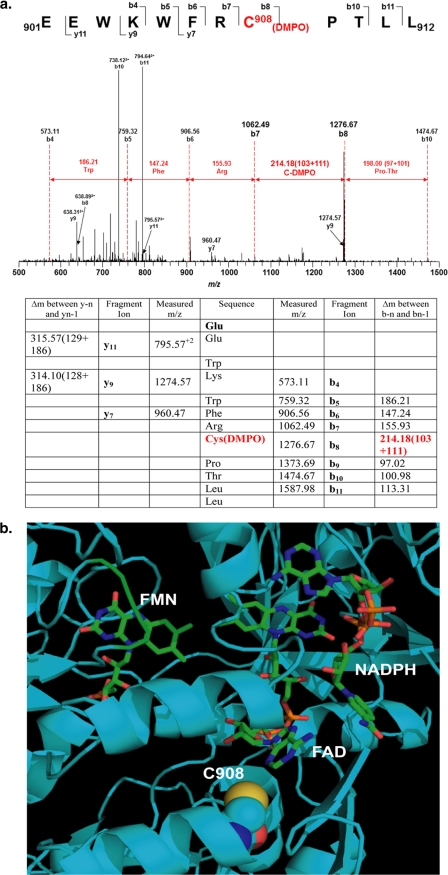
FIGURE 2.
Mass spectrometry and molecular modeling reveal that Cys-908 is the site for eNOS protein radical formation. a, LC-MS/MS analysis of eNOS protein radical formation. Protein radical formation was demonstrated to be on Cys-908 using DMPO as a trap. The molecular mass difference between fragment ions b7 and b8 demonstrated a mass shift of 111 Da compared with the native fragment ions; this allowed unequivocal assignment of the DMPO adduct to Cys-908. b, molecular modeling of human eNOS reductase domain. The three-dimensional structure of human eNOS reductase domain was generated using the reductase domain of rat neuronal NOS (Protein Data Bank code 1F20) by Swiss molecular modeling. Cys-908 (C908) is located at the interface of FAD and FMN domain. This residue is conserved throughout all mammalian species. Structural perturbation of this domain is expected when this residue is mutated to Ala allowing oxygen to access this pocket.