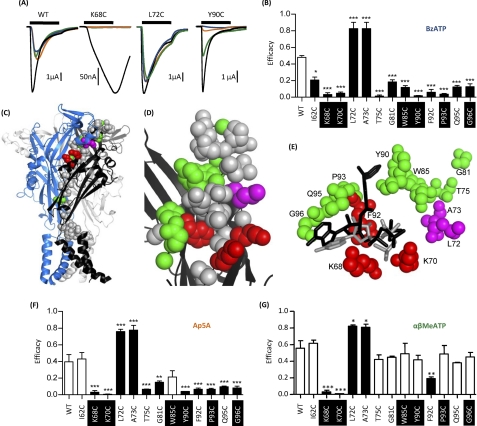
FIGURE 3.
Effect of cysteine point mutations on partial agonist efficacy. Partial agonist action was tested on WT and P2X1 receptor mutants expressed in oocytes by two-electrode voltage clamp (holding potential, −60 mV). A, representative recordings of currents evoked by partial agonists (100 μm) compared with control ATP (100 μm) (black) for WT and mutants K68C, L72C, and Y90C. K68C and Y90C both show a decrease in efficacy of BzATP (blue) compared with the variable sensitivity of AP5A (amber) and α,β-MeATP (green), with L72C having an increased efficacy of all of the partial agonists. B, summary of all mutants demonstrating significant changes in efficacy of BzATP. C, schematic representation of the P2X1 receptor homology model; mutants that modified BzATP efficacy are shown as spheres. Red, decrease in ATP potency and reduced BzATP efficacy; green, no effect on ATP potency and reduced efficacy; magenta, no effect on ATP potency and increased efficacy. D, higher magnification of the region around the proposed ATP binding pocket showing mutants that change BzATP efficacy (same coloring as in C). E, molecular docking of BzATP to the P2X1 receptor shows that BzATP can fit within the proposed ATP binding pocket. Docking is shown for both ATP (in gray) and BzATP (in black), and residues that modified BzATP efficacy are shown (same coloring as in C and D). F and G, the effect of AP5A and α,β-MeATP on the corresponding mutants. Significant changes in efficacy are shown as black bars. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Conserved residues are highlighted in black. Error bars, S.E.