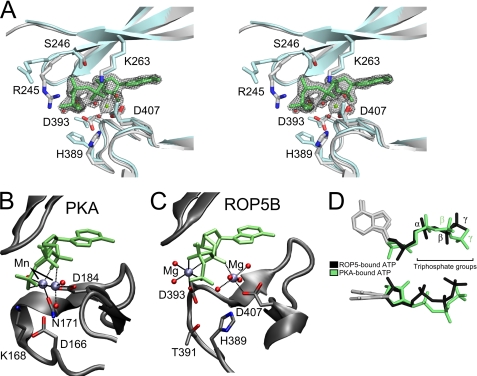
FIGURE 3.
ROP5 binds ATP in an unusual conformation. A, a stereo view of the pseudoactive site of ROP5BI is shown comparing the ATP-bound (gray) and unbound (blue) structures. Residues that make contact with ATP are shown as sticks. The ATP and its associated Mg2+ and waters are superposed with the 1.72-Å 2Fo − Fc electron density map contoured at 2.5 σ. The PKA (B) and ROP5BI (C) active sites are shown with bound ATP in an orientation to highlight the magnesium conformation. D, the ATP molecules bound to ROP5 (black) or PKA (green) have been overlaid to highlight triphosphate conformation.