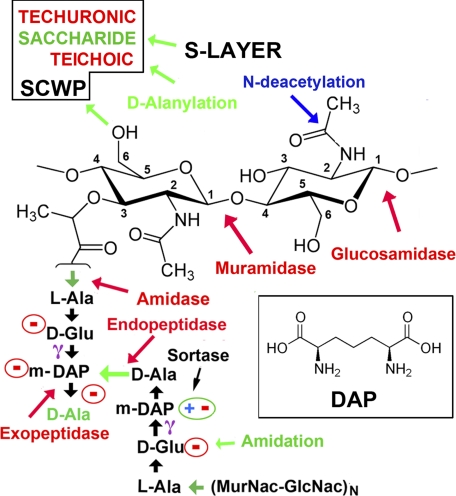
FIGURE 1.
Schematic of the PG type A1γ cross-link. Two (MurNAc-GlcNAc)N glycan chains are cross-linked by a pair of tetrapeptides, l-Ala-d-Glu-(meso-DAP)-d-Ala. The carboxyl group on a MurNac moiety forms an amide bond with the α-amino group of l-Ala. This bond is the target of amidase lysins. d-Glu uses its γ-carboxyl to makes an amide bond with m-DAP (structure of DAP in inset), leaving its α-carboxylate free. In the A1γ class, the bi-functional m-DAP makes a cross-peptide bond to the terminal d-Ala from the other peptide. The terminal noncross-linked d-Ala is often removed by a carboxypeptidase. In the other major class of PG, a lysine residue replaces DAP, and the transpeptide bond is formed via the side-chain Nζ (although this is typically not direct). Free charges are circled, giving a potential net charge of Z = −4 for the muropeptide bridge. However, species- and strain-specific modifications are common; typically one (not both) of the free acids (d-Ala or m-DAP) is enzymatically amidated. N-Deacetylation of either MurNac or GlcNAC would create a free amino group (positively charged) on the glycan. The cleavage positions of other common lysins are indicated (in red). SCWPs are typically attached via the C-6 hydroxyl group on MurNac. In B. anthracis, the S-layer attaches via the SCWP (16). Sortases may covalently link a number of proteins to the free carboxylate on DAP or other peptides in the muropeptide linkage.