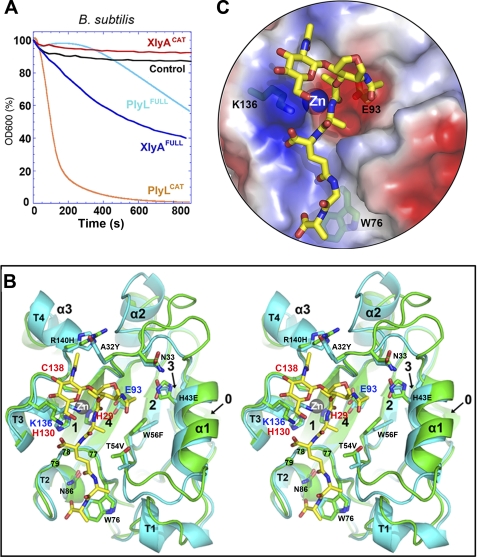
FIGURE 2.
Similar structures and distinct behaviors of two homologous amidase lysins, PlyL and XlyA. A, bactericidal activities against B. subtilis of full-length lysins and catalytic domains, as determined by decrease in light scattering of cells in suspension with time (see “Experimental Procedures”). Control is addition of no enzyme. B, stereo overlay of XlyACAT (cyan) and PlyLCAT (green), shown as schematics, with secondary structure elements labeled as in Fig. 3A. Selected protein side chains are shown as sticks (carbon atoms are cyan or green; others are colored by atom type). Residues in the 77–79 loop are shown as balls. A glycan-muropeptide is also modeled into the active site (carbon atoms are yellow). C, semitransparent surface representation, same view as B, showing fit of the muropeptide to the active site, and the locations of the key catalytic machinery (Lys-136, Zn2+, and Glu-93), as well as Trp-76, which may play a role in PG selectivity (53).