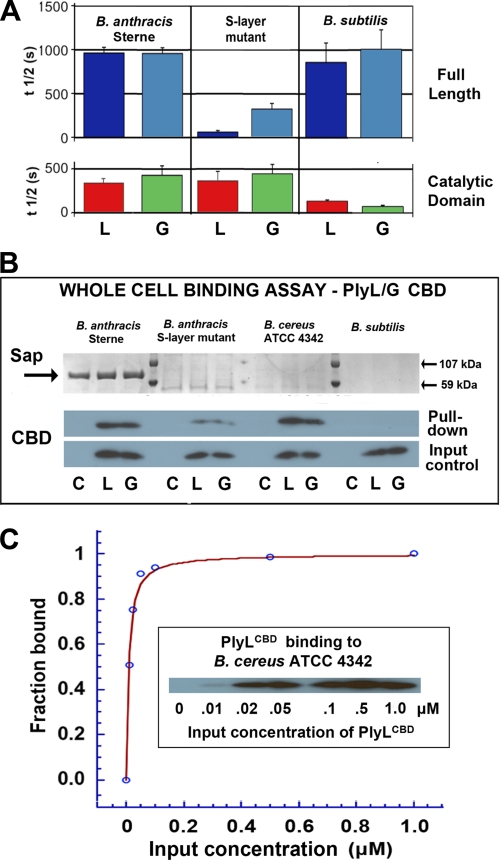
FIGURE 5.
Effect of the B. anthracis S-layer on lytic activity and whole cell binding by two homologous amidases, PlyL and PlyG. A, cell killing activities of full-length (top) or catalytic domain (bottom) of PlyL (“L”) and PlyG (“G”) against B. anthracis Sterne, B. anthracis (sap−) mutant deficient in S-layer synthesis, and B. subtilis. Lysin concentration was 0.4 μm in each case. B, whole-cell pulldown binding assays using His-tagged CBDs (1 μm) of PlyL (“L”) and PlyG (“G”) and mutant and wild-type B. anthracis, B. cereus 4342 (which has a similar PG/SCWP architecture but does not express an S-layer), and B. subtilis, which serves as a control (it has a different SCWP architecture and does not express an S-layer). Lower gels are Western blots using anti-His antibody (C = cell pellet alone). Upper gels show expression of Sap protein, with markers at 59 and 107 kDa. C, quantitative binding assay for PlyLCBD to live B. cereus cells (see “Experimental Procedures”). The derived Kd = 7.8 ± 0.8 nm; curve-fitting quality parameters are: χ2 = 0.0067; R = 0.996.