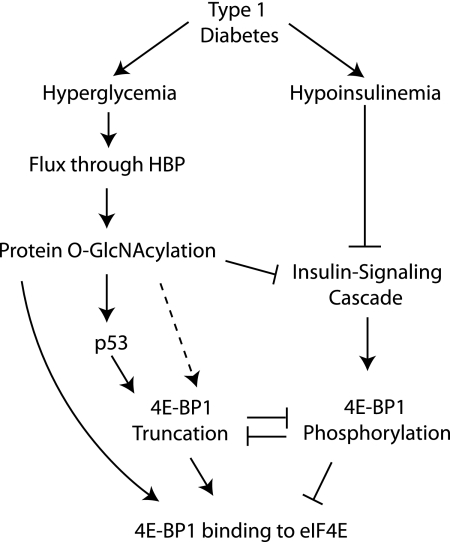
FIGURE 8.
Model for hyperglycemia-induced 4E-BP1 O-GlcNAcylation and truncation. Under diabetic conditions, a combination of hyperglycemia and hypoinsulinemia produce increased 4E-BP1 binding to eIF4E to inhibit the translation of mRNAs through a cap-dependent process. Hyperglycemia-driven increased flux through the hexosamine biosynthetic pathway (HBP) leads to O-GlcNAcylation of numerous proteins. Repressed insulin-signaling occurs in response to both hypoinsulinemia and O-GlcNAcylation of IRS-1 and Akt, leading to reduced phosphorylation of 4E-BP1 by the mTORC1 signaling pathway. Hypophosphorylated 4E-BP1 binds strongly to eIF4E, whereas phosphorylation leads to dissociation. Reductions in 4E-BP1 phosphorylation are associated with increased 4E-BP1 O-GlcNAcylation, which enhances the interaction of 4E-BP1 with eIF4E independent of the phosphorylation state. In addition, O-GlcNAcylation of p53 blocks its proteasomal degradation, potentially increasing p53-mediated truncation of 4E-BP1. Truncated 4E-BP1 is almost completely unphosphorylated and interacts strongly with eIF4E. Although phosphorylation of 4E-BP1 inhibits its truncation, it is possible that O-GlcNAcylation may serve a role in directly promoting truncation, thereby further enhancing its interaction with eIF4E (dashed line).