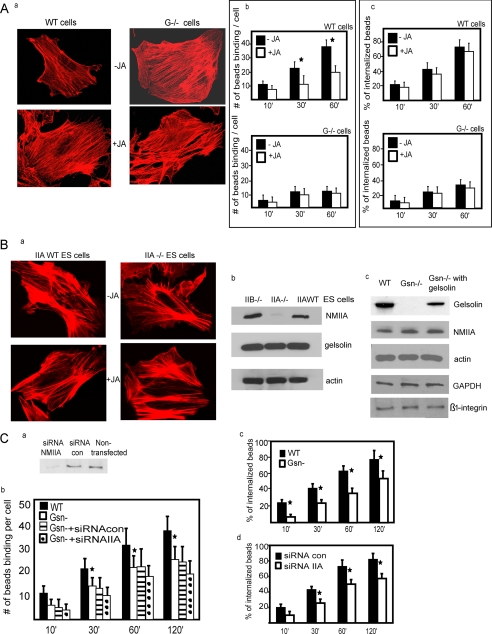
FIGURE 1.
A, a, gelsolin WT and gelsolin null cells with and without treatment with JA (500 nm) and stained with rhodamine-phalloidin. b, gelsolin WT and null cells were treated with JA and plated on collagen-coated beads. Data are means ± S.E. of bound collagen beads per cell (n = 100 cells/group). *, difference of p < 0.05. c, similarly, the % bead internalization was not different in the presence or absence of JA in gelsolin null cells and gelsolin WT cells. B, a, phalloidin-stained cells showed no difference in actin filament distribution in untreated or JA-treated cells. b, total cell lysates from NMIIB null, NMIIA null, and WT ES cells were immunoblotted for the indicated proteins. c, similarly, cell lysates from gelsolin WT, gelsolin null cells, and gelsolin null cells transfected with gelsolin cDNA show levels of indicated proteins. C, a, fibroblasts from gelsolin null mice transfected with NMIIA siRNA show >70% reduction of NMIIA protein levels compared with cells treated with non-targeted siRNA. b, the numbers of collagen beads binding per cell were significantly (p < 0.05) lower in gelsolin null cells and in gelsolin null cells transfected with siRNA NMIIA. c, in fibroblasts from WT mice treated with NMIIA or non-targeted siRNA as control, internalized beads were discriminated by quenching the fluorescence of extracellular bound beads with trypan blue. Fluorescent and quenched beads were counted in 60 cells/sample at each time point, and NMIIA knockdown cells showed reduced internalization for all time points (p < 0.02). d, collagen bead internalization assays on gelsolin WT and null cells.