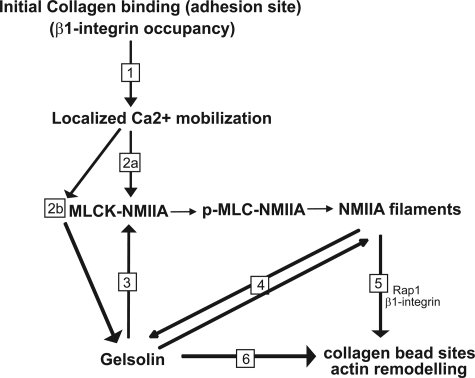
FIGURE 8.
Schematic diagram indicating the functional dependence of collagen phagocytosis on NMIIA interactions with gelsolin. 1, initial binding of collagen to β1 integrin at collagen bead adhesion sites. 2a, localized Ca2+ flux enhances phosphorylation of myosin light chain at serine 19 and increases NMIIA filament assembly. 2b, Ca2+ flux induces activation of gelsolin and MLCK, myosin light chain kinase. 3, gelsolin is required for MLC phosphorylation because the absence of [Ca2+]i in gelsolin null cells prevents the phosphorylation of NMIIA MLC required for myosin IIA filament assembly. 4, NMIIA filaments interact with gelsolin to provide anchorage for gelsolin at collagen bead binding sites. 5, NMIIA filaments provide Rap1 localization at bead sites and enable β1 integrin activation (12). 6, spatial localization of gelsolin by NMIIA filaments provides remodeling of actin filaments around bead binding sites, which is required for phagosome formation.