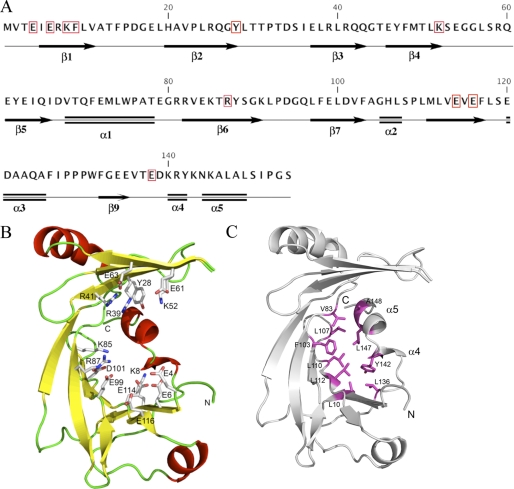
FIGURE 1.
Structure of NeuTTM. A, amino acid sequence and secondary structure elements are shown. The residues conserved among known CYTH proteins (9) are highlighted by red boxes. B, a schematic representation of the NeuTTM monomer is shown. Side chains of residues probably involved in substrate and divalent cation binding and/or catalysis are rendered as sticks and are labeled. C, stabilization of the open β-barrel structure by hydrophobic interactions with α-turn 4 and α-helix 5 is shown. The NeuTTM monomer is shown in a schematic representation (gray). The residues forming a hydrophobic patch that stabilizes the protein core are shown in magenta sticks and labeled.