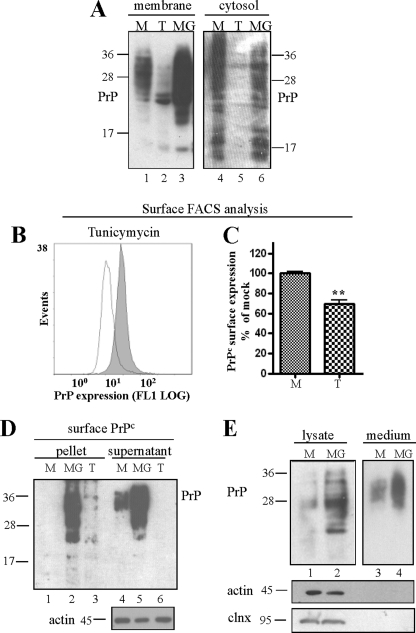
FIGURE 4.
PrP aggregates accumulating upon proteasomal inhibition increasingly localize at the cell surface. A, immunoblot of PrP-transfected HpL3-4 cells upon crude membrane fraction preparation. Tunicamycin (T) and MG132 (MG) treatment did not increase the amount of membrane-bound PrPc (compared with mock treated (M) cells). B and C, tunicamycin treatment (open line) resulted in reduced cell surface localization of PrPc when measured in surface FACS analysis with non-permeabilized HpL3-4 cells. D, transiently PrP-transfected HpL3-4 cells were biotinylated upon treatment with tunicamycin or MG132. Aggregated and insoluble PrP were separated by ultracentrifugation assay and precipitated with mAb 4H11. Surface-expressed, biotinylated PrP was monitored by probing blots with streptavidin. Equal protein loading was confirmed with actin (bottom panel). E, the amount of secreted PrP was measured in culture medium of mock and MG132-treated cells upon immunoblotting. Significantly more PrP was collected in the medium of cells treated with MG132. Cell contamination in the medium was excluded by probing cell lysates and media fraction with antibodies for non-secretory proteins calnexin and actin.