Abstract
Conjugation of Nedd8 to a cullin protein, termed neddylation, is an evolutionarily conserved process that functions to activate the cullin-RING family E3 ubiquitin ligases, leading to increased proteasomal degradation of a wide range of substrate proteins. Recent emerging evidence demonstrates that cellular neddylation requires the action of Dcn1, which, in humans, consists of five homologues designated as hDCNL1–5. Here we revealed a previously unknown mechanism that regulates hDCNL1. In cultured mammalian cells ectopically expressed hDCNL1 was mono-ubiquitinated predominantly at K143, K149, and K171. Using a classical chromatographic purification strategy, we identified Nedd4-1 as an E3 ligase that can catalyze mono-ubiquitination of hDCNL1 in a reconstituted ubiquitination system. In addition, the hDCNL1 N-terminal ubiquitin-binding domain is necessary and sufficient to mediate mono-ubiquitination. Finally, fluorescence microscopic and subcellular fractionation analyses revealed a role for mono-ubiquitination in driving nuclear export of hDCNL1. Taken together, these results suggest a mono-ubiquitination-mediated mechanism that governs nuclear-cytoplasmic trafficking of hDCNL1, thereby regulating hDCNL1-dependent activation of the cullin-RING E3 ubiquitin ligases in selected cellular compartments.
Keywords: Cell Compartmentation, Post-translational Modification, Protein Translocation, Ubiquitin Ligase, Ubiquitination, Dcn1, cullin-RING E3
Introduction
Nedd8 is an ubiquitin (Ub)3-like protein that primarily functions to modify the cullin family proteins, yielding an isopeptide linkage that joins the carboxyl end of Nedd8 glycine 76 (Gly-76) and the ϵ-amino group of a conserved cullin lysine residue (1). Cullins are molecular scaffolds that build multisubunit cullin-RING E3 Ub ligase (CRL) complexes (2–3). CRLs are generally organized in two distinct modules, capable of binding and positioning a substrate, as well as recruiting an E2 Ub-conjugating enzyme, respectively. Among the best-understood CRLs, SCF (Skp1-Cullin 1-F box protein; CRL1) is composed of four subunits, including cullin 1 (CUL1), Skp1, an F-box protein of substrate targeting function, and the ROC1/Rbx1 RING finger protein capable of recruiting an E2, such as Cdc34 or UbcH5 (4). Within SCF, the N terminus of CUL1 anchors the Skp1-F box protein heterodimer, whereas the cullin C terminus docks ROC1/Rbx1. Hence a substrate, once bound to the F-box protein, is positioned within close proximity of a RING domain-tethered E2-S∼Ub. Consequently, the attack of E2-S∼Ub by a substrate lysine results in the transfer of Ub to the target protein. However, in the unmodified state, SCF appears restrained due to the auto-inhibitory interactions between ROC1/Rbx1 and the CUL1 C-terminal tail (5). The conjugation of Nedd8 to CUL1 K720 activates SCF (1, 6), most likely by inducing drastic conformational changes in CUL1 that liberate ROC1/Rbx1 (7). Recent studies have provided cell-based evidence that neddylation-induced conformational changes stimulate CRL activities (8).
Early biochemical experiments have led to reconstitution of the Nedd8 conjugation reaction, termed neddylation, thereby elucidating a mechanistic framework that is similar to ubiquitination (9, 10). Neddylation commences with the ATP hydrolysis-driven formation of a thiol ester complex, linking the carboxyl end of Nedd8 Gly-76 to an active Cys residue in Nedd8 E1, which is formed by the APP-BP1/Uba3 heterodimer. A trans-thiolation reaction then follows to produce the Ubc12 (E2)-S∼Nedd8 thiol ester (11). Finally, the attack by a conserved cullin lysine, such as CUL1 K720, to Ubc12-S∼Nedd8 yields the cullin-Nedd8 isopeptide bond linkage. In these reconstitution studies, the cullin substrates are in complexes with ROC1/Rbx1 and importantly, the integrity of the RING finger is indispensable for neddylation (9). Moreover, it was observed that ROC1/Rbx1 binds to Ubc12 directly (12, 13). Given these findings, it was proposed that the ROC1/Rbx1 RING finger acts as an E3 for cullin neddylation (12, 13).
However, subsequent studies have suggested that cullin neddylation is subject to regulation through the action of Dcn1. Compelling genetic evidence pointed to a requirement for Dcn1 in neddylation in both Caenorhabditis elegans and budding yeast (14). In C. elegans, Dcn1 is necessary for post-meiotic degradation of MEI-1, a process mediated by the CRL3 E3 in a Nedd8-dependent fashion (14). The crystal structure of yeast Dcn1 revealed a conserved, C-terminally located PONY domain (15, 16). It was shown that in yeast, the acidic DAD patch of the Dcn1 PONY domain interacts with cullins and that this interaction appears critical for stimulating cullin neddylation in vitro (16). More recently, an elegant biochemical and structural study by Schulman and co-workers (17) has demonstrated that yeast Dcn1 acts as a co-E3 that facilitates ROC1/Rbx1 for neddylation.
In humans, five Dcn1 homologues were identified and they are designated as hDCNL1-5 (14, 18). Of hDCNL1–5, hDCNL1, and hDCNL2 closely resemble yeast Dcn1, containing both the N-terminal UBA (ubiquitin-binding domain) and C-terminal PONY domains. On the other hand, hDCNL3–5 only share the conserved PONY domain but containing distinct N termini. The complexity of the DCNL family proteins points to a possibility that mammals may employ diversified mechanisms to regulate Dcn1-dependent cullin neddylation, thereby altering CRL activities in response to developmental or environmental cues. Indeed, hDCNL1 (also named SCCRO) is highly amplified in tumors including squamous cell carcinomas (19) and is thought to be a risk factor for frontotemporal lobar degeneration (20). In contrast, the hDCNL3 level drops in liver, bladder, and renal tumors (21). It was recently shown that hDCNL3 localizes to the plasma membrane through a conserved N-terminal membrane motif (18). Interestingly, hDCNL1 appears critical for CUL1 neddylation in the nucleus (22). In this investigation, we discovered that ectopically expressed hDCNL1 is mono-ubiquitinated. Biochemical chromatographic and reconstitution experiments suggest Nedd4-1 as a likely E3 that catalyzes the mono-ubiquitination of hDCNL1. Finally, results from fluorescence microscopic and subcellular fractionation experiments point to a role for mono-ubiquitination in driving nuclear export of hDCNL1.
EXPERIMENTAL PROCEDURES
Plasmid Construction
The human hDCNL1 coding sequence was purchased from Invitrogen (UltimateTM ORF clone ID IOH12273). Using the Gateway® recombination system (Invitrogen), four expression vectors were created: pcDNA3.1-nV5-hDCNL1 and pDEST27-GST-hDCNL1 for expression in mammalian cell culture, as well as pDEST15-GST-hDCNL1 and pDEST17-His-hDCNL1 for expression in bacteria.
To express individual domains, we constructed pDEST15-GST-hDCNL1 (1–57) and pDEST15-GST-hDCNL1 (58–259). Site-directed mutagenesis (QuikChange®, Stratagene) was employed using pDEST15-GST-hDCNL1 as template and the truncations were recombined into Gateway® destination vectors. The primers for hDCNL1 (1–57) are: (5′)-GTACAAAAAAGCAGGCACCATGGGATCATTGGACAGGAAGAAG-3′) and (5′-CTTCTTCCTGTCCAATGATCCCATGGTGCCTGCTTTTTTGTAC-3′). The primers for hDCNL1 (58–259) are: (5′-CGAGA GAGTGTAAAATGATCATTGGACAGGAAGAAG-3′) and (5′-CTTCTTCCTGTCCAATGATCATTTTACACTCTCTCG-3′).
QuikChange® site-directed mutagenesis was also used to create the triple lysine mutant plasmid, pcDNA3.1-nV5-hDCNL1 3KR in two steps. In step 1, pcDNA3.1-nV5-hDCNL1 was used as template to generate pcDNA3.1-nV5-hDCNL1 K143R/K149R using a pair of mutagenic primers: (5′-GCCCAGATACCCAGGATGGAACAAGAATTGAGAGAACCAGGAGG-3′) and (5′-CCTCCTGGTTCTCTCAATTCTTGTTCCATCCTGGGTATCTGGGC-3′). In step 2, pcDNA3.1-nV5-hDCNL1 K143R/K149R was used as template using the K171R-mutagenic primers: (5′-GCAAAGAATCCAGGACAAAGAGGATTAGATCTAGAAATG-3′) and (5′-CATTTCTAGATCTAATCCTCTTTGTCCTGGATTCTTTGC-3′).
To carry out subcellular localization studies, we constructed pcDNA-DEST53-GFP-hDCNL1 using the Gateway® recombination system. We created pcDNA-DEST53-GFP-hDCNL1-UbΔG75G76 using a combination of site-directed mutagenesis and PCR (Expand High Fidelity PCR system, Roche). The primers for site-directed mutagenesis are: (5′-GCTGGGACAAAAAGTACAACAGTGGGTACCCAGCTTTCTTGTACAAAGTGG-3′) and (5′-CCACTTTGTACAAGAAAGCTGGGTACCCACTGTTGTACTTTTTGTCCCAGC-3′). The primers for PCR amplification of UbΔG75G76 are: (5′-CCCGGTACCATGCAGATCTTCGTGAAGACTCTG-3′) and (5′-CCCGGTACCTCATCTGAGACGGAGTACCAGGTGCAAG-3′). The engineered KpnI sites used for cloning are underlined. To construct pcDNA3.1-nV5-hDCNL1-UbΔG75G76, pcDNA-DEST53-GFP-hDCNL1-UbΔG75G76 was used as template to retrieve the sequence that encodes for hDCNL1-UbΔG75G76 through a PCR reaction using the primers: (5′-CACCATGAACAAGTTGAAATCATCGCAG-3′) and (5′-TCATCTGAGACGGAGTACCAGGTGCAAG-3′). The resulting PCR fragment was then subcloned into pENTR/d-TOPO, which was subsequently recombined with pcDNA3.1-nV5-DEST. All constructs were verified by DNA sequencing.
In Vitro Neddylation
For reactions shown in Fig. 1B, reaction mixture (20 μl) contained 50 mm Tris-HCl, pH7.4, 5 mm MgCl2, 2 mm ATP, 0.6 mm DTT, 0.1 mg/ml BSA, Nedd8 (1.5 μm), APP-BP1/Uba3 (7.5 nm), ROC1-CUL1 (324–776) (0.25 μm), and Ubc12 (in amounts as specified). After incubation for 10 min at room temperature, the reaction products were separated by 4–20% SDS-PAGE, followed by anti-Flag immunoblot analysis to visualize Flag-CUL1 (324–776). The levels of reaction products were quantified by Odyssey® infrared imager (LI-COR).
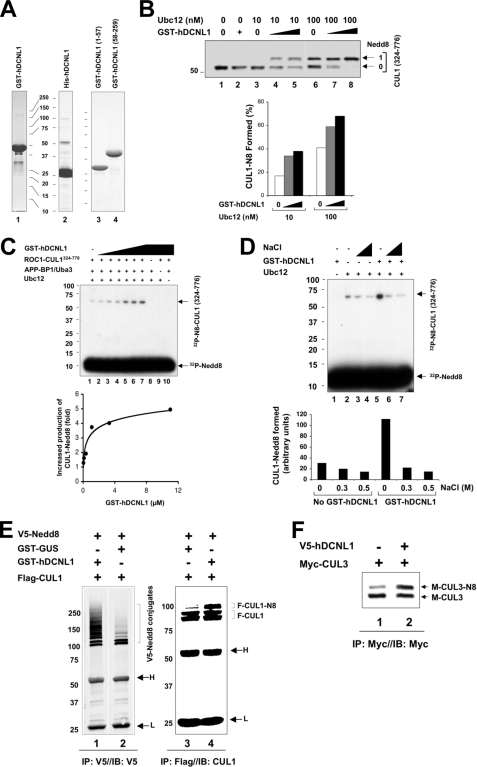
FIGURE 1.
Stimulation of cullin neddylation by hDCNL1. A, Coomassie staining analysis of purified hDCNL1 in full-length or truncated forms. B and C, activation of CUL1 neddylation by hDCNL1 in vitro. The effects of GST-hDCNL1 on neddylation in vitro were measured using either Nedd8 (panel B) or 32P-Nedd8 (panel C) as described under “Experimental Procedures.” The reaction products are visualized by immunoblot analysis (panel B) or autoradiogram (panel C). The quantification is presented graphically. D, hDCNL1-mediated activation of neddylation is sensitive to salt. The neddylation reaction was carried out as in panel C. The effect of salt (0.3 or 0.5 m NaCl) on the reaction is shown by autoradiogram and graph. E, stimulation of CUL1 neddylation by hDCNL1 in vivo. HEK293 cells were co-transfected with vectors expressing Flag-CUL1 and GST-hDCNL1 or GST-Gus. At 48 h post-transfection, cells were harvested and extracts (500 μg) were immunoprecipitated and analyzed by immunoblot analysis to detect V5-Nedd8 conjugates, Flag-CUL1 or its neddylated forms. F-CUL1-N8, Flag-CUL1-Nedd8. F-CUL1, Flag-CUL1. H, IgG heavy chain. L, IgG light chain. F, hDCNL1 stimulates in vivo neddylation of CUL3. The effect of V5-hDCNL1 on the neddylation of Myc-CUL3 was examined by transfection experiment as described for panel E. M-CUL3-N8, Myc-CUL3-Nedd8. M-CUL3, Myc-CUL1.
For reactions shown in Fig. 1, C and D, 32P-Nedd8 was used for neddylation. For this purpose, Nedd8 was initially phosphorylated in a reaction mixture (100 μl) that contained PK-Nedd8 (35 μg), 40 mm Tris-HCl, pH 7.4, 12 mm MgCl2, 2 mm NaF, 50 mm NaCl, 25 μm ATP, 50 μCi of [γ-32P]ATP, 0.1 mg/ml BSA, and 5 units of cAMP kinase (Sigma). The reaction was incubated at 37 °C for 30 min. To conjugate 32P-Nedd8 to CUL1, 32P-Nedd8 (1 μl) was incubated in a reaction (30 μl) that contained 50 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 2 mm ATP, 0.6 mm DTT, 0.1 mg/ml BSA, APP-BP1/Uba3 (3.3 nm), Ubc12 (3 nm), and ROC1-CUL1324–776 (0.25 μm), in the presence or absence of GST-hDCNL1 (1.2 μm or as indicated). The incubation was at 37 °C for 60 min. The reaction products were separated by 4–20% SDS-PAGE, quantified by phosphoimager analysis, and presented graphically.
Mapping in Vivo Ubiquitination Sites for hDCNL1
To map the hDCNL1 ubiquitination sites, we developed a procedure for affinity-purification of hDCNL1 in a stable HEK293 cell line (GD11) that was engineered to constitutively express GST-hDCNL1. The GD11 cell line was generated using a protocol as described previously (23). HEK293 cells, maintained in growth medium (DMEM supplemented with 10% bovine serum and 100 μg/ml of penicillin-streptomycin (Invitrogen)), were plated at an approximate density of 1 × 106 cells per plate (10 cm). At 20–24 h after plating, pDEST27TM-GST-hDCNL1 (8 μg) was co-transfected with a plasmid containing the puromycin selection marker (2 μg) using Fugene® 6 transfection reagent (Roche). At 48 h post-transfection, cells were trypsinized, diluted at a ratio of 1:5, 1:50, or 1:500 and subsequently plated onto 15 cm plates with growth medium containing puromycin (2.5 μg/ml). The selection medium was changed daily to remove dead cells in the subsequent 3 days and then every third day for another 2 weeks. Well-isolated colonies, becoming visible 10–15 days after the initial puromycin treatment, were picked by suction using a sterile 1 ml pipette tip and were placed into individual wells of 24-well plates containing the selection medium. The positive clones were identified by screening for expression of GST-hDCNL1 via immunoblot analysis.
50 150-cm plates of GD11 cells were grown in the presence of selection medium until confluent. After harvest, cell extracts (100 mg of protein, 2 mg/ml) were adsorbed to glutathione beads (2 ml) by rocking overnight at 4 °C. The beads were than washed with 40 ml of Buffer A (15 mm Tris (pH 7.4), 0.5 m NaCl, 0.35% Nonidet P-40, 5 mm EDTA, 5 mm EGTA, 1 mm PMSF, 2 μg/ml of antipain, and 2 μg/ml of leupeptin), and 20 ml of Buffer B (25 mm Tris (pH 7.5), 1 mm EDTA, 0.01% Nonidet P-40, 10% glycerol, 1 mm DTT, 0.1 mm PMSF, 2 μg/ml of antipain, and 2 μg/ml of leupeptin) plus 50 mm NaCl). The bound proteins were eluted with 10 ml of Buffer B plus 20 mm glutathione then concentrated ∼40-fold using an Amicon Ultra concentrator (Millipore). A sample of the eluate was subject to 4–20% SDS-PAGE and visualized with Coomassie Blue. The slow-migrating species of GST-hDCNL1 was excised and analyzed by mass spectrometry using methods as described (24, 25).
Reconstitution of hDCNL1 Mono-ubiquitination with HeLa Cell Extracts
Reaction mixture (20 μl) contained 40 mm CrPO4, pH7.7, 1 μg of creatine phosphokinase, 5 mm MgCl2, 2 mm ATP, 0.6 mm DTT, 1 μg of purified GST-hDCNL1 (or GST), 0.5 μg of UbcH5c, E1 (15 ng), and 50 μg of HeLa extract proteins, prepared as described previously (26). After incubation at 37 °C for 60 min, the reaction mixture was adsorbed to glutathione beads (20 μl). Following extensive washing, the bound proteins were eluted by SDS, separated by 4–20% SDS-PAGE, and visualized by immunoblot analysis using anti-GST antibody.
In Vitro Isolation and Identification of the hDCNL1 E3
The hDCNL1 E3 was isolated from HeLa extracts based on its ability to catalyze the mono-ubiquitination of hDCNL1, as monitored by immunoblot assays. HeLa cell extracts (11 mg of protein/ml, 120 ml) were prepared as described previously (26) and maintained in buffer B plus 25 mm NaCl. The extracts were chromatographed through a Mono Q HR 10/10 (Amersham Biosciences) in 4 batches. Bound protein was eluted by 160 ml-gradient of 0.025–0.7 m NaCl. Active fractions, eluted at around 200 mm NaCl, were pooled (90 mg of protein), and concentrated ∼20-fold using an Amicon Ultra concentrator (Millipore). The resulting protein (51 mg) was then chromatographed by FPLC using a Superose 6 HR 10/30 gel filtration column (Amersham Biosciences) in 13 batches. Active fractions were tracked, pooled (32 mg), and re-chromatographed through Mono Q HR 10/10 (Amersham Biosciences) in 2 batches. Bound protein was eluted by a 160-ml gradient of 0.025–0.5 m NaCl. Active fractions, eluted again at around 200 mm NaCl, were pooled (10 mg of protein), and concentrated ∼30-fold using an Amicon Ultra concentrator (Millipore). All the eluted materials were subject to glycerol gradient sedimentation with buffer B containing 15–35% of glycerol plus 25 mm NaCl in an ultracentrifuge (Beckman) with a SW50.1 rotor at 45,000 rpm for 20 h.
To identify hDCNL1 E3, the active fractions from the glycerol gradient were pooled and concentrated (5.4 mg of total protein). Samples were then loaded onto 4–20% SDS-PAGE and stained with Coomassie Blue. Each band was then excised and analyzed via mass spectrometry (MS/MS) as described previously (24, 25).
Reconstitution of hDCNL1 Mono-ubiquitination by Nedd4-1
Reaction mixture (20 μl) contained 50 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 2 mm ATP, 0.6 mm DTT, 0.1 mg/ml BSA, GST-hDCNL1, or His-hDCNL1 (35 pmol), Ub (1.5 μg), E1 (15 ng), UbcH5c (0.5 μg), and Nedd4-1 (50 ng or as indicated). Production of mono-ubiquitinated products, resolved on 4–20% SDS-PAGE, was quantified by Odyssey® infrared imager (LI-COR) and is presented graphically.
Analysis of Subcellular Localization of hDCNL1
U2OS cells (1 × 105) were grown on glass coverslips in 6-well dishes overnight and transfected with pcDNATM-DEST53-GFP-hDCNL1 or pcDNATM-DEST53-GFP-hDCNL1-UbΔG75G76. At 48-h post-transfection, the cells attached to the coverslips were washed with phosphate-buffered saline (PBS), fixed for 15 min with 2% paraformaldehyde, then mounted on slides using Prolong® antifade reagent with DAPI (Invitrogen). Where indicated, at 24 h post-transfection, cells were treated with Leptomycin B (LMB, Calbiochem) with a concentration of 1, 5, or 25 ng/ml and cultured for another 24 h before fixing. Fluorescent microscopy was performed on a Zeiss Axioplan2 IE epi-fluorescence microscope. In each set, >150 cells were examined and the percentage of cells with GFP-hDCNL1 or GFP-hDCNL1-UbDG75G76 enriched in nucleus or cytoplasm was calculated and shown graphically.
For nuclear/cytoplasmic fractionation experiments 10-cm dishes of HEK293T cells were transfected with pcDNA3.1-nV5-hDCNL1 or pCDNA3.1-nV5-hDCNL1-UbΔG75G76 plasmid using FuGENE® HD (Roche) for 48 h as per manufacturer's instructions. The cells were then harvested and extracted using NE-PER® nuclear and cytoplasmic extraction reagent (Thermo Scientific) according to manufacturer's instructions. The extracts were then analyzed by immunoblotting.
Others
Purified Nedd4-1 was prepared as described previously (27). Other enzymes and proteins were prepared as described: PK-Ub (28); ROC1-CUL1324–776, Nedd8, Ubc12, UbcH5c (29); and PK-Nedd8 (26). E1 and APP-BP1/Uba3, were purchased from Boston Biochem (Cambridge, MA). The commercial sources for antibodies are: anti-Nedd4-1 (Upstate Biotechnology), anti-laminA/C (Millipore), and anti-tubulin (Sigma).
RESULTS
Activation of Cullin Neddylation by hDCNL1 in Vitro and in Vivo
We sought to determine whether hDCNL1 activates cullin neddylation. For this purpose, we expressed and purified recombinant hDCNL1 (Fig. 1A, lanes 1 and 2). To assess the effects of purified hDCNL1 in neddylation, we employed the reconstituted neddylation assay that consists of purified Nedd8, APP-BP1/Uba3 (as E1), Ubc12 (as E2), and ROC1-CUL1324–776 (as the RING-tethered substrate complex). Previous studies have established ROC1-CUL1324–776 as an efficient substrate for in vitro neddylation (10, 26, 29) and moreover, recent work has revealed a direct interaction between this complex and hDCNL1 (16). As shown, more than 40% of CUL1 substrate was converted to the neddylated form when 100 nm of Ubc12 was used (Fig. 1B, lane 6; graph). Decreasing the concentration of Ubc12 by a factor of 10 diminished neddylation (Fig. 1B, lane 3). Under this condition, addition of GST-hDCNL1 at concentrations 4 or 12 times greater than CUL1 markedly stimulated neddylation (Fig. 1B, lanes 3–5; graph). The stimulatory effect was also observed at high concentrations of Ubc12, albeit to a lesser extent (Fig. 1B, lanes 6–8; graph). To more quantitatively evaluate the hDCNL1-mediated activation of CUL1 neddylation in vitro, we employed the reconstituted neddylation assay with radioactive Nedd8, as it was established previously (26). The results revealed that GST-hDCNL1 stimulated neddylation by a factor of five in the presence of low concentration of Ubc12 (Fig. 1C). In addition, the hDCNL1-mediated activation of CUL1 neddylation was inhibited by addition of salt (Fig. 1D). This finding is in keeping with the previous observations that electrostatic interactions between yeast Dcn1 and cullin play critical roles in establishing contacts between these components (16). Of note, His-hDCNL1 activated CUL1 neddylation as well (data not shown). In all, these results demonstrate that hDCNL1 activates CUL1 neddylation directly, with the most pronounced effect seen in the presence of low concentration of Ubc12. However, the extent of the hDCNL1-mediated stimulation was significantly lower than that observed with yeast Dcn1 (17), suggesting that additional factor(s) may be required for the action of hDCNL1 in neddylation.
To evaluate the effects of hDCNL1 in cellular neddylation, we first co-transfected HEK293 cells with vectors expressing V5-Nedd8 and GST-hDCNL1 or GST-Gus (as a control). Immunoprecipitation and immunoblot analysis revealed that expression of GST-hDCNL1, but not GST-Gus, led to increased accumulation of high molecular weight V5-Nedd8 conjugates (Fig. 1E, lane 1). This finding suggested that hDCNL1 activates conjugation of Nedd8 to cellular proteins. When overexpressed in HEK293 cells, GST-hDCNL1, but not GST-Gus, increased the yield of neddylated Flag-CUL1 (Fig. 1E, lane 4). Similarly, co-expression of V5-hDCNL1 stimulated the neddylation of Myc-CUL3 (Fig. 1F). Under these conditions, overexpression of V5-hDCNL1 did not affect the cellular neddylation of CUL2 or CUL4A (data not shown). Future work is required to determine whether hDCNL1 selectively stimulate the neddylation of a subset of cullins. Of note, significant cross-reactivity of commercially available anti-cullin antibodies has precluded a reliable assessment of the effect of hDCNL1 in the neddylation of endogenous cullins. Taken together, these observations suggest a direct role for hDCNL1 in activating in vitro neddylation of CUL1 and in vivo neddylation of CUL1 and CUL3.
hDCNL1 Is Mono-ubiquitinated
When expressed in HEK293T cells, GST-hDCNL1 exhibited two forms: one corresponding to unmodified species and the other migrating significantly slower (Fig. 2A, lanes 1 and 2). Consistent with this finding, two forms of V5-hDCNL1 were also detected in transfected HEK293T cells (Fig. 2B, lanes 1 and 2). To determine if this modified hDCNL1 species resulted from mono-ubiquitination, HEK293T cells were co-transfected with vectors expressing GST-hDCNL1 and HA-Ub. The results of glutathione pull-down and immunoblot experiments showed that the co-expression of HA-Ub led to the formation of a GST-hDCNL1 species that corresponded to GST-hDCNL1-HA-Ub (Fig. 2C, lanes 1 and 3). This species disappeared in the absence of HA-Ub (Fig. 2C, lanes 2 and 4). Of note, the modified form of GST-hDCNL1 that migrated slightly faster than GST-hDCNL1-HA-Ub (Fig. 2C, compare lanes 1 and 2), represented mono-ubiquitinated GST-hDCNL1 formed by the endogenous Ub, because it reacted with anti-ubiquitin antibody (Fig. 2C, lane 6). Similar observations were made with co-expression of V5-hDCNL1 and HA-Ub (Fig. 2D). In contrast, no mono-neddylated hDCNL1 was detected upon co-expression with tagged Nedd8 (data not shown). These results demonstrate that ectopically expressed hDCNL1 is mono-ubiquitinated in cultured cells.
FIGURE 2.
hDCNL1 is mono-ubiquitinated. A and B, hDCNL1 is modified. HEK293T cells were transfected with vectors expressing GST-hDCNL1 or V5-hDCNL1. GST-Gus was used as control. At 48 h post-transfection, cells were harvested. Extracts (50 μg) were used for direct immunoblot analysis. For pull-down experiments, extracts (500 μg) were used for glutathione matrix purification or immunoprecipitation by anti-V5 antibodies. The respective tagged proteins, separated by 4–20% SDS-PAGE, were detected by anti-GST (panel A) or anti-V5 (panel B) antibodies. G-Gus, GST-Gus. G-hDCNL1, GST-hDCNL1. Glut PD, glutathione bead pull down. H, IgG heavy chain. L, IgG light chain. C and D, hDCNL1 is mono-ubiquitinated. HEK293T cells were co-transfected with vectors expressing HA-Ub and GST-hDCNL1 or V5-hDCNL1. HA-XPB was used as control. Extracts (500 μg) were subject to glutathione matrix pull down (panel C) or immunoprecipitation by V5-antibodies (panel D). The precipitates were separated by 4–20% SDS-PAGE, followed by immunoblot using antibodies as specified. The hDCNL1 and its modified forms are marked on gel.
To map the ubiquitination sites on hDCNL1, we generated a HEK293-based cell line that constitutively expresses GST-hDCNL1 and prepared a large amount of mono-ubiquitinated GST-hDCNL1 by affinity purification (Fig. 3A). Mass spectrometry analysis of the modified GST-hDCNL1 fraction revealed three ubiquitination sites on the PONY domain: K143, K149, and K171 (Fig. 3A). Replacement of K143, K149, and K171 by Arg reduced mono-ubiquitination of GST-hDCNL1 or V5-HDCNL1 by 80% (Fig. 3, B and C). Taken together, these results established unequivocally that in mammalian cultured cells recombinant hDCNL1 is mono-ubiquitinated predominantly at K143, K149, and K171. At the present time, the poor quality of our hDCNL1 antibody precludes the assessment of mono-ubiquitination of the endogenous form of hDCNL1.
FIGURE 3.
Identification of the hDCNL1 ubiquitination sites. A, large scale preparation of GST-hDCNL1-Ub for mass spectrometric analysis. Shown is the Coomassie stain of GST-hDCNL1 expressed and purified from GD11 cells as described under “Experimental Procedures.” The slow-migrating form of GST-hDCNL1 was excised and subject to mass spectrometry, which revealed that K143, K149, and K171 are the major ubiquitination sites. B and C, replacement of hDCNL1 K143, K149, and K171 by arginine impairs mono-ubiquitination in vivo. Vectors encoding for GST-hDCNL1 3KR or V5-hDCNL1 3KR were constructed to express a mutant form of hDCNL1 in which K143, K149, and K171 were replaced by arginine. Following transfection into HEK293T cells along with wild type expression vector, cells were harvested and extracts (500 μg) were subject to glutathione matrix pull-down (panel B) or immunoprecipitation by V5-antibodies (panel C). The precipitates were separated by 4–20% SDS-PAGE, followed by immunoblot using antibodies as specified.
Nedd4-1 Supports Mono-ubiquitination of hDCNL1 in Vitro
We reconstituted the mono-ubiquitination of hDCNL1 in vitro by incubating purified GST-hDCNL1 with HeLa cell extracts and UbcH5c (as E2), in the presence of ATP-regenerating system. As revealed by immunoblot analysis, this incubation converted a significant portion of GST-hDCNL1 to a slower-migrating species, in a manner that depended on both HeLa extracts and UbcH5c (Fig. 4A, compare lane 4 with lanes 2 and 3). In contrast, the same treatment did not cause modification of GST (Fig. 4A, lane 1). These observations suggest that HeLa extracts contain an E3 activity that catalyzes the mono-ubiquitination of hDCNL1.
FIGURE 4.
Isolation and identification of the hDCNL1 E3. A, HeLa extracts catalyze the mono-ubiquitination of hDCNL1. Reaction was carried out and analyzed as described under under “Experimental Procedures.” B, flow chart outlines a strategy for the isolation and identification of hDCNL1 E3. See “Experimental Procedures” for details. C, molecular weight estimation of the hDCNL1 E3. Top, gel filtration analysis. A Stokes radius standard curve, generated using molecular size markers, is shown. The position of the hDCNL1 E3 is marked, which corresponds to a Stokes radius of 5.58 nm. Bottom, glycerol gradient analysis. A sedimentation S value standard curve, generated using molecular size markers, is shown. The position of the hDCNL1 E3 is marked, which corresponds to an S value of 5.47 S. D, Nedd4-1 is co-migrating with the hDCNL1 mono-ubiquitination activity. Aliquots (3 μl) of the indicated glycerol gradient fractions were analyzed by the hDCNL1 mono-ubiquitination assay. The protein peak of Nedd4-1 is revealed by immunoblot analysis with antibodies specific for Nedd4-1. Load: an aliquot of the starting material for glycerol gradient sedimentation. Thy: thyroglobulin (Mr = 670,000); Cat: catalase (Mr = 240,000); BSA: bovine serum albumin (Mr = 66,000).
To isolate the HeLa cell E3 activity responsible for the hDCNL1 mono-ubiquitination, we developed a strategy that combined chromatography-based partial purification, molecular weight estimation via sizing chromatography and glycerol gradient sedimentation, mass spectrometry to identify E3 candidates, and verification of E3 by immunoblotting as well as ubiquitination assays (Fig. 4B). We reasoned that given the availability of gene sequences encoding for RING/HECT E3s, analysis of only partially purified E3 fraction by mass spectrometry and by molecular weight estimation would yield information sufficient for the identification of hDCNL1 candidate E3s in vitro.
The results of gel filtration and glycerol gradient sedimentation experiments revealed that the hDCNL1 E3 activity has Stokes Radius of 5.58 nm and Svedberg value of 5.47S (Fig. 4C), respectively. When calculated based on the formula by Siegel and Monty (30), the apparent molecular mass of the hDCNL1 E3 is 126 kDa. Mass spectrometry of the glycerol gradient fraction of the hDCNL1 E3 yielded four E3 ligases with the molecular mass in the range of 100–150 kDa, including RING finger protein 160, Nedd4-1, c-CBL, and SMURF1. When tested by immunoblot analysis, Nedd4-1 was found to track with the hDCNL1 mono-ubiquitination activity across the glycerol gradient fractions (Fig. 4D). In contrast, neither c-CBL nor SMURF1 co-migrated with the hDCNL1 mono-ubiquitination activity (data not shown). These results suggest Nedd4-1 as a likely candidate E3 for hDCNL1.
To confirm Nedd4-1 as hDCNL1 E3 in vitro, the recombinant E3 protein was tested for its ability to catalyze the mono-ubiquitination of GST-hDCNL1. As shown by titration (Fig. 5A) and kinetic (Fig. 5B) experiments, purified Nedd4-1 catalyzed the mono-ubiquitination of GST-hDCNL1, converting nearly 50% substrate into ubiquitinated products. However, a significant lag time (∼15 min) appeared to be required for the modification reaction. Similar observations were made with V5-hDCNL1, albeit with lower levels of mono-ubiquitination (Fig. 5C). Of note, repeated attempts involving pre-incubation of Nedd4-1 with or without hDCNL1 did not significantly diminish the lag time period for the mono-ubiquitination (data not shown). Thus, first charging Nedd4-1 by Ub does not accelerate the mono-ubiquitination of hDCNL1 (see “Discussion”). Furthermore, it should be cautioned that future genetic work is required to determine whether Nedd4-1 acts as the physiological E3 for hDCNL1.
FIGURE 5.
Recombinant Nedd4-1 catalyzes the mono-ubiquitination of hDCNL1. Purified Nedd4-1 (5–80 ng) was used for E3 titration (panels A and C) and time course (panel B) experiments in vitro with GST-hDCNL1 (panels A and B) or His-hDCNL1 (panel C), as described under “Experimental Procedures.” Production of mono-ubiquitinated products was quantified and is presented graphically. In panel D, GST-hDCNL1 (50 ng), in the forms of full-length (lanes 1 and 2) or truncations (amino acids 1–57, lanes 3 and 4; amino acids 58–259, lanes 5 and 6), was subject to the ubiquitination assay in the presence of or absence of Nedd4-1.
The mono-ubiquitination activity was mapped to the N terminus of hDCNL1, as GST-hDCNL1 (1–57) (see Fig. 1A, lane 3), but not GST-hDCNL1 (58–259) (see Fig. 1A, lane 4), was mono-ubiquitinated by Nedd4-1-1 in vitro (Fig. 5D). Given that hDCNL1 (1–57) contains the UBA domain (spanning amino acids 8–45), these observations suggest that the hDCNL1 UBA domain is necessary and sufficient to mediate the mono-ubiquitination reaction. In agreement of this observation, GST-hDCNL1 (58–259), which lacks the UBA domain, was not mono-ubiquitinated in HEK293T cells (data not shown).
Mono-ubiquitination Drives Nuclear Export of hDCNL1
Mono-ubiquitination can effectively drive nuclear export of a target protein such as p53 (31, 32). It was shown that in-frame fusion of an Ub moiety with p53 is sufficient to induce nuclear export (31, 32). We adopted the Ub fusion strategy to evaluate a possible role for mono-ubiquitination in the regulation of the nuclear-cytoplasmic trafficking of hDCNL1. For this purpose, U2OS cells were transfected with vectors expressing GFP-hDCNL1 or GFP-hDCNL1-UbΔGG (UbΔGG eliminates its utilization as donor for conjugation). Fluorescence microscopy revealed that in over 90% of GFP-hDCNL1-expressing cells, the recombinant protein was enriched in the nucleus (Fig. 6A, graph), suggesting that hDCNL1 is predominantly nuclear. In contrast, in ∼85% of cells expressing GFP-hDCNL1-UbΔGG, the Ub fusion protein was located mostly in the cytoplasm (Fig. 6A, graph), implicating that Ub fusion redistributed hDCNL1 from nucleus to cytoplasm.
FIGURE 6.
Mono-ubiquitination drives the nuclear export of hDCNL1. A, Ub fusion translocates hDCNL1 from nucleus to cytoplasm. Top, fluorescence imaging of representative cells expressing GFP-hDCNL1 fused with or without Ub. Bottom, bar graphs indicate the percentage of cells expressing GFP-hDCNL1 or GFP-hDCNL1-UbΔGG that was enriched in nucleus, cytoplasm, or nucleus/cytoplasm. In each set, more than 150 cells were analyzed. Note that the graph integrates the results of three independent experiments, with error bars for the calculated standard deviation. B, LMB inhibits the cytoplasmic accumulation of GFP-hDCNL1-UbΔGG. The cells expressing GFP-hDCNL1 or GFP-hDCNL1-UbΔGG were treated with increasing doses of LMB. Bar graphs indicate the percentage of cells expressing GFP-hDCNL1 or GFP-hDCNL1-UbΔGG that was enriched in nucleus, cytoplasm, or nucleus/cytoplasm. In each set, more than 150 cells were analyzed. C, cell fractionation analysis. HEK293T cells were transfected with vectors expressing V5-hDCNL1 or V5-hDCNL1-UbΔGG. Following separation of nucleus and cytoplasm as described under “Experimental Procedures,” the respective fractions were analyzed by immunoblot analysis with indicated antibodies. While lanes 1 and 3 contain cytoplasmic fraction, lanes 2 and 4 are loaded with nuclear proteins. Lamin A/C and tubulin are widely used nuclear and cytoplasmic markers, respectively.
To determine whether Ub fusion promotes nuclear export of hDCNL1, the GFP-hDCNL1-UbΔGG-expressing U2OS cells were treated with or without leptomycin (LMB), which inhibits the activity of the CRM-1 nuclear exporter. The results revealed that LMB reduced the cytoplasmic localization of hDCNL1-UbΔGG and concomitantly, increased its nuclear distribution in a dose-dependent manner (Fig. 6B). Of note, in a significant portion of LMB-treated cells, GFP-hDCNL1-UbΔGG exhibited both nuclear and cytoplasmic localization (Fig. 6B). Thus, it is evident that LMB inhibited nuclear export of hDCNL1-UbΔGG.
To confirm the role of Ub fusion in nuclear export of hDCNL, subcellular fractionation experiments were performed to monitor the distribution of V5-hDCNL1 or V5-hDCNL1-UbΔGG in transfected HEK293T cells. V5-hDCNL1 and V5-hDCNL1-UbΔGG were expressed similarly at levels that were ∼4-times higher than the endogenous hDCNL1 (data not shown). As revealed by direct immunoblot analysis, while V5-hDCNL1 was enriched in the nucleus (Fig. 6C, lanes 1 and 2), V5-hDCNL1-UbΔGG was found accumulated in the cytoplasm (Fig. 6C, lanes 3 and 4). On average, the ratio of nuclear/cytoplasmic form of V5-hDCNL1 and V5-hDCNL1-UbΔGG is 3:1 and 1:2, respectively. The identity of V5-hDCNL1 and hDCNL1-UbΔGG was confirmed by immunoprecipitation (data not shown).
At the present time, the unavailability of a quality hDCNL1 antibody has precluded our assessment of whether mono-ubiquitination promotes the nuclear export of the endogenous form of hDCNL1. It should be mentioned that despite repeated efforts, we have been unable to detect any significant effects of mono-ubiquitination on hDCNL1-mediated activation of CUL1 neddylation in vitro (data not shown). Altogether, these results suggest a primary role for mono-ubiquitination in promoting the nuclear export of hDCNL1.
DISCUSSION
Mono-ubiquitination of hDCNL1 by Nedd4-1 in Vitro
We observed extensive mono-ubiquitination of hDCNL1 in transfected cells (Fig. 2), with K143, K149, and K171 as major ubiquitination sites (Fig. 3). HeLa cell extracts contained a major hDCNL1 mono-ubiquitination activity (Fig. 4A), which was subsequently determined to be Nedd4-1 through chromatographic purification and mass spectrometry (Fig. 4, B–D). Recombinant Nedd4-1 catalyzed the mono-ubiquitination of hDCNL1 (Fig. 5, A–C). However, hDCNL1 appears not to contain a conserved WW domain, which is the determinant recognized by the Nedd4-1 class E3 (33).
We suggest that the mono-ubiquitination of hDCNL1 by Nedd4-1 may proceed in a mechanism independent of WW domain-mediated interactions. Note that the hDCNL1 N-terminal UBA domain is indispensable for mono-ubiquitination (Fig. 5D), suggesting a critical role for Ub binding in this modification reaction. On this note, several groups have recently shown that a few HECT domain-containing E3s (RSP5, Nedd4–2, and Smurf) recognizes Ub directly (34–36). On the basis of these findings, we propose that the mono-ubiquitination commences with the binding of Ub to the hDCNL1 UBA domain. The hDCNL1-linked Ub may be recognized by Nedd4-1, which initiates mono-ubiquitination. Alternatively, hDCNL1 may utilize “coupled mono-ubiquitination” mechanism, initially proposed for the mono-ubiquitination of eps15, a UIM-containing protein, by the Nedd4-1 family E3 ligases (37, 38). In this model, Nedd4-1 would first undergo mono-ubiquitination and then the modified E3 would bind to the UBA domain of hDCNL1 via interactions mediated by the E3-linked Ub moiety. Either model could explain a significant lag time observed for the ubiquitination of hDCNL1 by Nedd4-1 in our kinetic analysis (Fig. 5B), because the hDCNL1 UBA domain-mediated interactions are likely of low affinity.
The ubiquitination sites (K143, K149, and K171) were mapped to the hDCNL1 PONY domain (Fig. 3). However, the hDCNL1 N-terminal fragment (1–57), lacking any of these ubiquitination sites, was found to be ubiquitinated by Nedd4-1 in vitro (Fig. 5D), suggesting a considerable flexibility in the selection of lysine receptors for ubiquitination.
Nuclear Export of hDCNL1 Driven by Mono-ubiquitination
C-terminal fusion of Ub to hDCNL1 resulted in its translocation from nucleus to cytoplasm (Fig. 6A), in a manner that was sensitive to the treatment with nuclear export inhibitor (Fig. 6B). Thus, mono-ubiquitination appears to drive nuclear export of hDCNL1, a function that has been well documented in the p53 nuclear-cytoplasmic trafficking (31, 32). However, while mono-ubiquitination of p53 is thought to unmask the nuclear export signal (32), it remains to be determined how mono-ubiquitination promotes nuclear export of hDCNL1. Inspection of hDCNL1 sequence has failed to identify conserved nuclear export or import signal sequence. It is intriguing to note that the conserved nuclear export sequence is enriched with hydrophobic residues (39). Given that Ub I44 is critical for p53 nuclear export (32), it is possible that the Ub I44/L8 hydrophobic patch, if it remains surface-exposed upon conjugation to hDCNL1, might act as nuclear export signal that interacts with export machinery.
It remains to be elucidated the cellular mechanisms that trigger the mono-ubiquitination of hDCNL1. The governing factor for this modification reaction is likely to be the interaction between the hDCNL1 UBA domain and Ub, or Nedd4-1-linked Ub. Such an interaction may be enhanced by yet-to-be determined mechanisms under conditions that favor activation of CRLs in the cytoplasm. Another important factor is the availability and accessibility of Nedd4-1. Nedd4-1 was shown to localize in the cytoplasm, mainly in the perinuclear region and cytoplasmic periphery (40). It was proposed that Nedd4-1 could translocate into the nucleus under certain cellular conditions, which induce conformational change exposing the nuclear localization signal on the surface of Nedd4-1 (40). Future study is required to assess the physiological role of Nedd4-1 in the regulation of hDCNL1 mono-ubiquitination and subcellular distribution.
In summary, our work suggests a role for the N-terminal UBA domain in the mono-ubiquitination of hDCNL1 to drive its nuclear export. Interestingly, a recent study has suggested that the UBA domain-lacking hDCNL3 utilizes a conserved N-terminal lipid-modified site to direct its localization to the plasma membrane (18). Together, these findings are consistent with a theme that conserved and yet distinct N termini play a directing role in the subcellular distribution of the hDCNL family proteins. The differentially dispatched hDCNL proteins may act to up-regulate various cullin-RING E3 ligase activities, thereby eliminating specific protein targets in selected cellular compartments.
Acknowledgments
We thank J. Hurwitz for regents. Fluorescent microscopy was performed at the MSSM-Microscopy Shared Research Facility, supported with funding from NIH-NCI shared resources grant (1 R24 CA095823-01) and NSF major research instrumentation grant (DBI-9724504).
This study was supported by Public Health Service Grants CA140451 (to C. P.), GM74830 (to L. H.), as well as GM61051 and CA095634 (to Z.-Q. P.). This work was also supported by a post-doctoral training grant from NCI, National Institutes of Health (to K. W.) and by American Cancer Society Fund (ACS-RSG-07-081-01-MGO-01) and Geoffrey Beene Cancer Research fund (to X. J.).
- Ub
- ubiquitin
- LMB
- leptomycin
- CRL
- cullin-RING E3 Ub ligase
- CUL
- cullin.
REFERENCES
- 1. Pan Z. Q., Kentsis A., Dias D. C., Yamoah K., Wu K. (2004) Oncogene 23, 1985–1997 [DOI] [PubMed] [Google Scholar]
- 2. Petroski M. D., Deshaies R. J. (2005) Nat. Rev. Mol. Cell Biol. 6, 9–20 [DOI] [PubMed] [Google Scholar]
- 3. Sarikas A., Hartmann T., Pan Z. Q. (2011) Genome Biol. 12, 220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu K., Kovacev J., Pan Z. Q. (2010) Mol. Cell 37, 784–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamoah K., Oashi T., Sarikas A., Gazdoiu S., Osman R., Pan Z. Q. (2008) Proc. Natl. Acad. Sci. 105, 12230–12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saha A., Deshaies R. J. (2008) Mol. Cell 32, 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duda D. M., Borg L. A., Scott D. C., Hunt H. W., Hammel M., Schulman B. A. (2008) Cell 134, 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boh B. K., Smith P. G., Hagen T. (2011) J. Mol. Biol. 409, 136–145 [DOI] [PubMed] [Google Scholar]
- 9. Kamura T., Conrad M. N., Yan Q., Conaway R. C., Conaway J. W. (1999) Genes Dev. 13, 2928–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu K., Chen A., Pan Z. Q. (2000) J. Biol. Chem. 275, 32317–32324 [DOI] [PubMed] [Google Scholar]
- 11. Huang D. T., Walden H., Duda D., Schulman B. A. (2004) Oncogene 23, 1958–1971 [DOI] [PubMed] [Google Scholar]
- 12. Dharmasiri S., Dharmasiri N., Hellmann H., Estelle M. (2003) EMBO J. 22, 1762–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morimoto M., Nishida T., Nagayama Y., Yasuda H. (2003) Biochem. Biophys. Res. Commun. 301, 392–398 [DOI] [PubMed] [Google Scholar]
- 14. Kurz T., Ozlü N., Rudolf F., O'Rourke S. M., Luke B., Hofmann K., Hyman A. A., Bowerman B., Peter M. (2005) Nature 435, 1257–1261 [DOI] [PubMed] [Google Scholar]
- 15. Yang X., Zhou J., Sun L., Wei Z., Gao J., Gong W., Xu R. M., Rao Z., Liu Y. (2007) J. Biol. Chem. 282, 24490–24494 [DOI] [PubMed] [Google Scholar]
- 16. Kurz T., Chou Y. C., Willems A. R., Meyer-Schaller N., Hecht M. L., Tyers M., Peter M., Sicheri F. (2008) Mol. Cell 29, 23–35 [DOI] [PubMed] [Google Scholar]
- 17. Scott D. C., Monda J. K., Grace C. R., Duda D. M., Kriwacki R. W., Kurz T., Schulman B. A. (2010) Mol. Cell 39, 784–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyer-Schaller N., Chou Y. C., Sumara I., Martin D. D., Kurz T., Katheder N., Hofmann K., Berthiaume L. G., Sicheri F., Peter M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12365–12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarkaria I., O-charoenrat P., Talbot S. G., Reddy P. G., Ngai I., Maghami E., Patel K. N., Lee B., Yonekawa Y., Dudas M., Kaufman A., Ryan R., Ghossein R., Rao P. H., Stoffel A., Ramanathan Y., Singh B. (2006) Cancer Res. 66, 9437–9444 [DOI] [PubMed] [Google Scholar]
- 20. Villa C., Venturelli E., Fenoglio C., Clerici F., Marcone A., Benussi L., Gallone S., Scalabrini D., Cortini F., Serpente M., Martinelli Boneschi F., Cappa S., Binetti G., Mariani C., Rainero I., Giordana M. T., Bresolin N., Scarpini E., Galimberti D. (2009) Eur. J. Neurol. 16, 870–873 [DOI] [PubMed] [Google Scholar]
- 21. Ma T., Shi T., Huang J., Wu L., Hu F., He P., Deng W., Gao P., Zhang Y., Song Q., Ma D., Qiu X. (2008) Cancer Sci. 99, 2128–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang G., Kaufman A. J., Ramanathan Y., Singh B. (2011) J. Biol. Chem. 286, 10297–10304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dias D. C., Dolios G., Wang R., Pan Z. Q. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16601–16606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones J., Wu K., Yang Y., Guerrero C., Nillegoda N., Pan Z. Q., Huang L. (2008) J. Proteome Res. 7, 1274–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang L., Wang X., Yamoah K., Chen P. I., Pan Z. Q., Huang L. (2008) J. Proteome Res. 7, 4914–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu K., Yamoah K., Dolios G., Gan-Erdene T., Tan P., Chen A., Lee C. G., Wei N., Wilkinson K. D., Wang R., Pan Z. Q. (2003) J. Biol. Chem. 278, 28882–28891 [DOI] [PubMed] [Google Scholar]
- 27. Wang X., Shi Y., Wang J., Huang G., Jiang X. (2008) Biochem. J. 414, 221–229 [DOI] [PubMed] [Google Scholar]
- 28. Tan P., Fuchs S. Y., Chen A., Wu K., Gomez C., Ronai Z., Pan Z. Q. (1999) Mol. Cell 3, 527–533 [DOI] [PubMed] [Google Scholar]
- 29. Wu K., Chen A., Tan P., Pan Z. Q. (2002) J. Biol. Chem. 277, 516–527 [DOI] [PubMed] [Google Scholar]
- 30. Siegel L. M., Monty K. J. (1966) Biochim. Biophys. Acta. 112, 346–362 [DOI] [PubMed] [Google Scholar]
- 31. Li M., Brooks C. L., Wu-Baer F., Chen D., Baer R., Gu W. (2003) Science 302, 1972–1975 [DOI] [PubMed] [Google Scholar]
- 32. Carter S., Bischof O., Dejean A., Vousden K. H. (2007) Nat. Cell Biol. 9, 428–435 [DOI] [PubMed] [Google Scholar]
- 33. Sudol M., Hunter T. (2000) Cell 103, 1001–1004 [DOI] [PubMed] [Google Scholar]
- 34. French M. E., Kretzmann B. R., Hicke L. (2009) J. Biol. Chem. 284, 12071–12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamadurai H. B., Souphron J., Scott D. C., Duda D. M., Miller D. J., Stringer D., Piper R. C., Schulman B. A. (2009) Mol. Cell 36, 1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogunjimi A. A., Wiesner S., Briant D. J., Varelas X., Sicheri F., Forman-Kay J., Wrana J. L. (2010) J. Biol. Chem. 285, 6308–6315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fallon L., Bélanger C. M., Corera A. T., Kontogiannea M., Regan-Klapisz E., Moreau F., Voortman J., Haber M., Rouleau G., Thorarinsdottir T., Brice A., van Bergen En Henegouwen P. M., Fon E. A. (2006) Nat. Cell Biol. 8, 834–842 [DOI] [PubMed] [Google Scholar]
- 38. Woelk T., Oldrini B., Maspero E., Confalonieri S., Cavallaro E., Di Fiore P. P., Polo S. (2006) Nat. Cell Biol. 8, 1246–1254 [DOI] [PubMed] [Google Scholar]
- 39. Sorokin A. V., Kim E. R., Ovchinnikov L. P. (2007) Biochemistry 72, 1439–1457 [DOI] [PubMed] [Google Scholar]
- 40. Anan T., Nagata Y., Koga H., Honda Y., Yabuki N., Miyamoto C., Kuwano A., Matsuda I., Endo F., Saya H., Nakao M. (1998) Genes Cells 3, 751–763 [DOI] [PubMed] [Google Scholar]