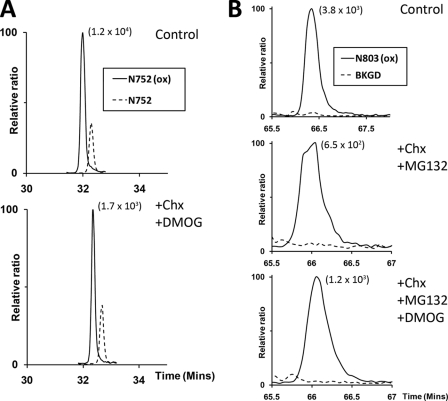
FIGURE 1.
Assessment of asparagine hydroxylation reversal on FIH targets by cycloheximide and DMOG treatment. A, extracted ion chromatograms (m/z 772.71 and 778.02) corresponding to unhydroxylated (dashed line) and hydroxylated (solid line) forms of the tryptic Rabankyrin-5 peptide containing the target Asn752 residue (N752, SGCDVNSPRQPGANGEGEEEAR [M + 3H]3+) bearing a single missed cleavage. Nano-UPLC-MSE chromatography analysis illustrating no significant change in the proportion of hydroxylated to unhydroxylated peptide signals upon inhibition of FIH-mediated catalysis and new protein synthesis: control (upper panel, 77%); +CHX/+DMOG (lower panel, 76%). B, extracted ion chromatograms (m/z 1061.16) corresponding to the mass of the hydroxylated (solid line) form of the tryptic HIF-1α peptide containing the target Asn803 residue (N803, LLGQSMDESGLPQLTSYDCEVNAPIQGSR [M + 3H]3+). Background traces (BKGD, dashed line). Nano-UPLC-MSE analysis demonstrates no appreciable reduction in the extent of hydroxylation upon inhibition of FIH-mediated catalysis and new protein synthesis in the context of the Rabankyrin-5/HIF1-CAD fusion protein: control (upper panel, >98%), +CHX/+MG132 (middle panel, >98%), +CHX/+MG132/DMOG (lower panel, >98%). The signal intensity of reference peaks is indicated in parentheses on the chromatograms.