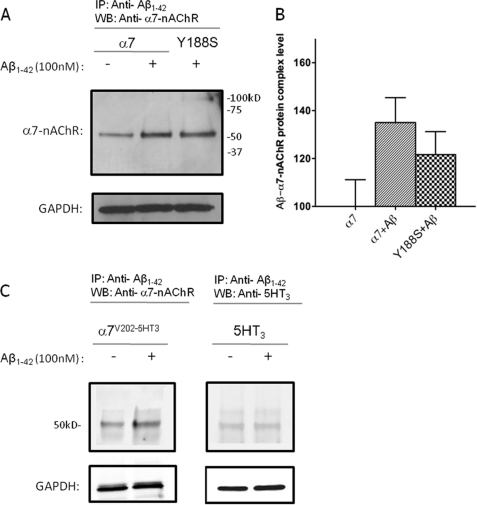
FIGURE 5.
Coimmunoprecipitation of WT and mutant α7-nAChRs with Aβ. Representative immunoblots for α7-nAChR or Y188S α7-nAChR (A), chimeric α7V202-5HT3A receptor (C, left panel) and 5HT3A receptor following immunoprecipitation (IP) (C, right panel) with anti-Aβ antibodies. Differentiated NG108-15 cells expressing WT α7-nAChRs or Y188S α7-nAChRs, chimeric α7V202-5HT3A receptors, or 5HT3A receptors were incubated for 30 min without (control) or with 100 nm Aβ1–42. Solubilized lysates from the cultures were then subjected to immunoprecipitation using a combination of two anti-Aβ monoclonal antibodies, followed by immunoblotting (WB) for α7-nAChR, chimeric α7V202-5HT3A receptor or 5HT3A receptor, respectively. Immunodetection of GAPDH in the cell lysates was used to control for total protein levels. The level of background signal for α7-nAChR found in the absence of Aβ using this protocol was similar to that found previously (15). To rule out contamination of the α7-nAChR signal with heavy chains from the anti-Aβ antibodies, which would have a very similar Mr (approximately 55 kDa) to that of α7-nAChR, a control was performed without use of the primary anti-α7 antibody and revealed only a very weak background signal. Blots are representative of three experiments. B, quantification of the results shown in A for four separate cultures, normalized to WT.