FIGURE 7.
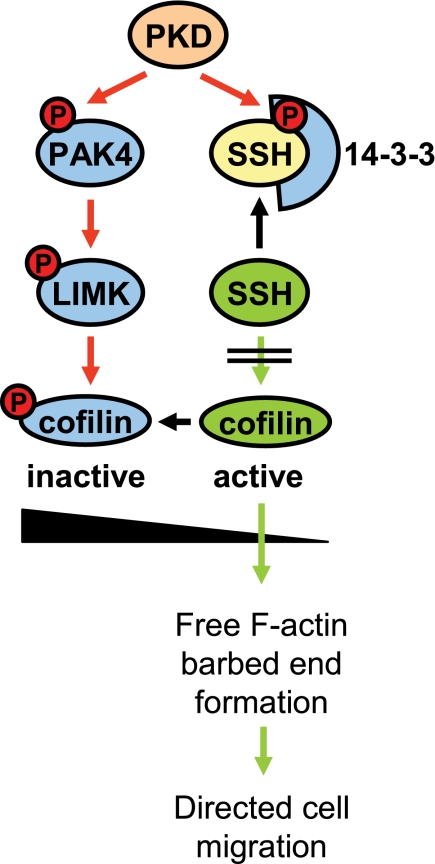
Proposed model in which PKD regulates cofilin activity via both PAK4/LIMK and SSH pathways. PKD enzymes regulate cofilin activity by phosphorylation of SSH1L at Ser-978. This generates a binding motif for 14-3-3 proteins which sequester SSH1L in the cytosol. Here, we put forward that PKD isoenzymes also regulate cofilin activity via a second pathway where PKD phosphorylates and activates PAK4. This mediates activation of the PAK4 downstream target LIMK, resulting in further increased levels of inactive phospho-cofilin. This confers to the observed complete block of cofilin-induced barbed end formation and actin incorporation at the leading edge and may explain the effects of active PKD on inhibiting directed cell migration.