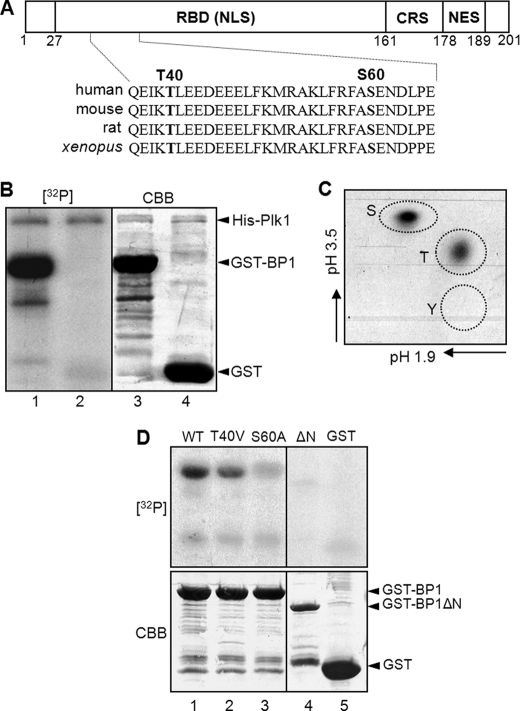
FIGURE 3.
RanBP1 is phosphorylated by Plk1. A, a schematic structure of mammalian RanBP1. RBD, Ran-binding domain containing the nuclear localization signal (NLS); CRS, cytoplasmic retention signal; NES, nuclear export signal. Threonine 40 (T40) and serine 60 (S60) residues in the N-terminal region are conserved in various species as Plk1 substrate-consensus sequences. B, purified RanBP1 proteins were phosphorylated by Plk1 in vitro. An in vitro kinase assay was conducted as described under “Experimental Procedures.” GST-RanBP1 was phosphorylated by Plk1 ([32P] lane 1), but GST alone was not ([32P] lane 1). The total protein amounts were shown by Coomassie Brilliant Blue staining (CBB; bottom). C, phosphorylation site(s) of RanBP1 in B was analyzed by phosphoamino acid analysis. S, phosphoserine; T, phosphothreonine; Y, phosphotyrosine. D, phosphorylation signals of mutant constructs of RanBP1 ([32P] lanes 2–4) decreased more than that of wild type RanBP1 ([32P] lane 1). Protein amounts were shown by Coomassie Brilliant Blue staining (CBB; bottom).