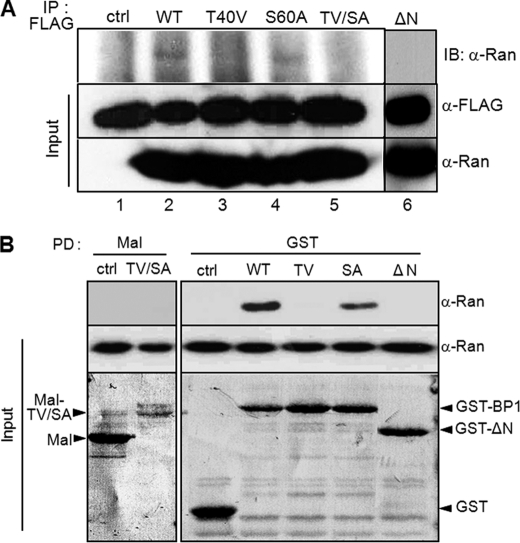
FIGURE 4.
Thr-40 phosphorylation of RanBP1 is essential for interaction with Ran. A, in vivo protein binding assay by immunoprecipitation. Various RanBP1 constructs containing FLAG tag were transfected for 24 h and then subjected to nocodazole treatment for 10 h to arrest in early mitosis. RanBP1s overexpressed were immunoprecipitated using anti-FLAG antibody (IP: FLAG). Bound Ran proteins were detected by Western blot (IB: α-Ran). The protein amounts are shown in the bottom panels (Input). B, in vitro protein binding assay by pull-down using fusion proteins. Various RanBP1 mutants were constructed as fusion proteins using maltose-binding protein (Mal) (lanes 1 and 2) or glutathione S-transferase (GST) (lanes 3–7) and were purified by pull-down of appropriate beads. Bound Ran proteins were detected by anti-Ran antibody (top, α-Ran). The protein amounts are shown at the bottom (Input).