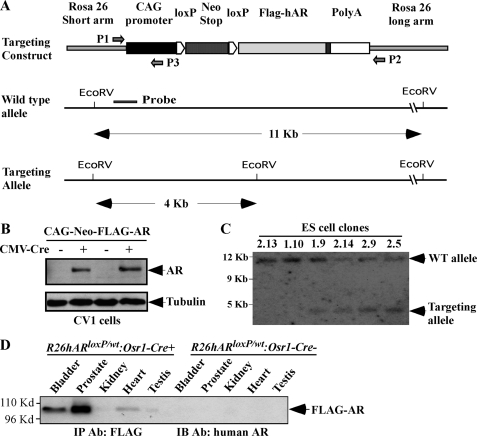
FIGURE 1.
Generating the AR conditional transgenic mice. A, a scheme of the conditional human AR transgene targeting construct is shown. A PGK-neomycin cassette with flanked loxP sites (LSL cassette) was inserted between the CAG promoter and a FLAG-tagged human AR coding sequence containing a nine-polyglutamine repeat tract. The DNA fragment isolated from the short arm region used as the probe in the Southern blot is marked as a solid line. The primers used for genotyping are marked with arrows. B, CV-1 cells were transfected with the targeting vector plasmid in the presence or absence of CMV-Cre expression vector to assess the activation of the AR transgene expression through the loxP/Cre recombination. Western blot was performed on cell lysates using the antibody against the human AR or β-tubulin. The expression of FLAG-tagged AR protein was detected in cells co-transfected with CMV-Cre vectors, demonstrating that a loxP/Cre recombination can result in the deletion of the LSL cassette and activation of AR transgene expression. C, Southern blot analysis was performed to examine ES cells transfected with the targeting vectors. Genomic DNA was digested by EcoRV and hybridized to a 32P-labeled probe (represented in Fig. 1a) located on the short arm. The wild type allele and targeting allele were differentiated by size and labeled. In four clones, two bands of expected size, 11.5 and 3.8 kb, were detected, representing the endogenous and targeted ROSA26 locus, respectively. D, whole protein lysates were isolated from different mouse tissues of 16-week-old R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/wt:Osr1-Cre− mice. Equal amounts of protein lysates were subjected to immunoprecipitation (IP) with FLAG antibody and then analyzed by Western blotting with a specific antibody against the human AR to detect the specific expression of the human AR transgene. IB, immunoblot.