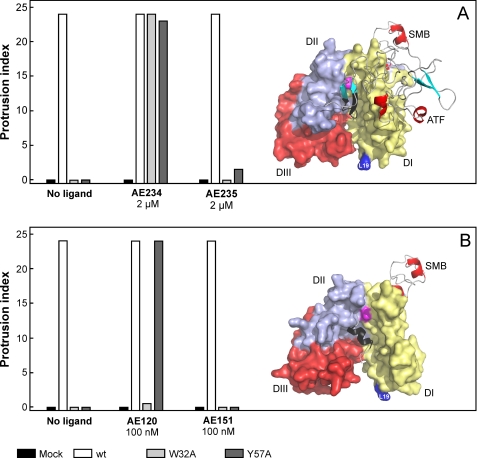
FIGURE 4.
Two different peptide antagonists behave differently as surrogates for stimulation of uPAR-dependent lamellipodia formation. Panel A shows that 2 μm AE234 is capable of inducing robust lamellipodia formation in HEK293 cell lines transfected with uPARW32A or uPARY57A. A scrambled, non-binding version of this peptide (AE235) does not induce this phenomenon. AE234 is a cyclic peptide surrogate of the receptor binding β-hairpin in uPA, where it mimics the region comprising residues 21–30 (shown in black in the model). This region of uPA is almost completely buried in the ATF-uPAR-SMB complex (17). In this schematic, uPAR is shown in a surface representation (DI, yellow; DII, light blue; DIII, red), whereas ATF and SMB are shown as ribbon representations. The position of Leu19 in uPAR is highlighted (blue) to facilitate comparison with data shown in Figs. 5 and 6. Panel B shows that AE120 only is capable of inducing lamellipodia in HEK293 cells transfected with uPARY57A, whereas HEK293 cells expressing uPARW32A are refractory to this stimulation. Its scrambled non-binding version (AE151) is inactive in both transfectants. AE120 is a linear peptide antagonist of the uPA-uPAR interaction, and the crystal structure of uPAR in complex with a truncated analog of AE120 (15) reveals that this antagonist engages the same region as the receptor binding β-hairpin in uPA (see the model). Nevertheless, it stabilizes a notably more open conformation of uPAR relative to that stabilized by ATF. The SMB is added in this schematic merely to illustrate the position of the vitronectin-binding epitope on uPAR. The position of Tyr57 in the central ligand binding cavity at the DI-DII interface is shown in magenta in both figures.