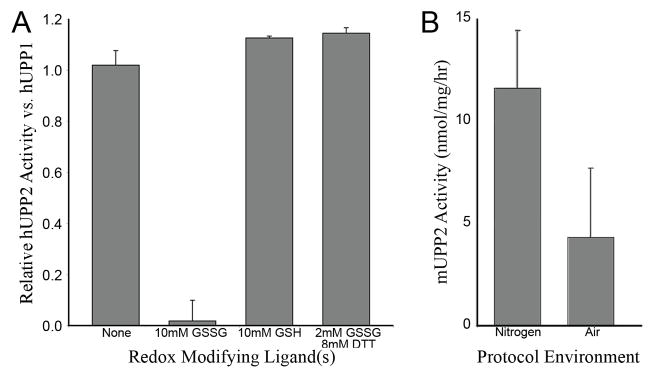
Fig. 5.
Redox sensitivity of UPP2. (A) The relative activity of recombinant hUPP2, normalized to hUPP1, upon exposure to various redox modifying compounds is graphed. hUPP2 activity is inactivated upon exposure to oxidized glutatione (GSSG) and slightly enhanced by reduced glutathione (GSH). Other reducing agents, such as dithiothreitol (DTT), can protect hUPP2 from GSSG inactivation, suggesting the observed effect is due to its redox properties and not through some other mechanism of molecular inhibition. (B) The measured activity of native mouse UPP2 is graphed, varying the composition of atmosphere used during enzyme isolation from liver homogenates and subsequent activity analysis. These results obtained with native enzyme confirm the oxidation sensitivity of this family of proteins.