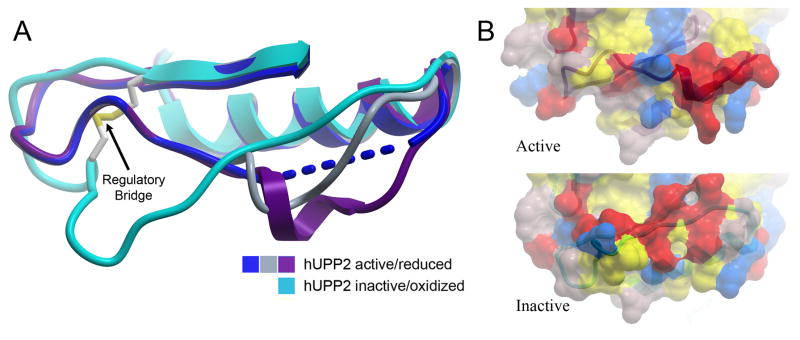
Fig. 6.
Long-range structural consequences of the state of the regulatory disulfide bridge. (A) Of six independent protein chains in the asymmetric unit of crystals of active hUPP2, four are too disordered for modeling in part of the loop region preceding the disulfide bridge (blue). The remaining two chains reveal partially helical structure (grey & purple), due to stabilization through protein-protein contacts with crystallographic-symmetry-related domains. These conformations differ substantially from the redox inactivated structure of hUPP2, in which this region of the protein is flat and elongated (turquoise). (B) Surface representation of the same region, colored by polarity and comparing active and inactive structures, illustrates the degree to which this face of the enzyme is altered, depending upon its redox state.