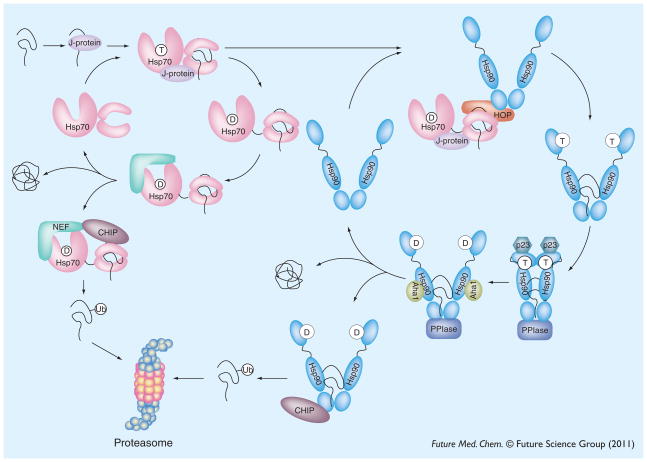
Figure 2. Model of heat shock protein 70–heat shock protein 90 cycling.
Unfolded or misfolded substrates enter the chaperone cycle by engaging in interactions with J-proteins, which recruit Hsp70 family chaperones. The ATPase cycle of Hsp70 can result in substrate folding, triage through the proteasome or ‘hand-off’ to the Hsp90 system. The ATPase cycle of Hsp90 refines folding and stabilizes the active form of many kinases and transcription factors. In addition, the Hsp90 cycle makes triage decisions, through CHIP-mediated ubiquitination and degradation.
CHIP: C-terminal Hsp70 interacting protein; HOP: Hsp70/Hsp90 organizing protein; Hsp: Heat shock protein; NEF: Nucleotide exchange factor; PPIase: Peptidyl-prolyl cis-trans isomerase.