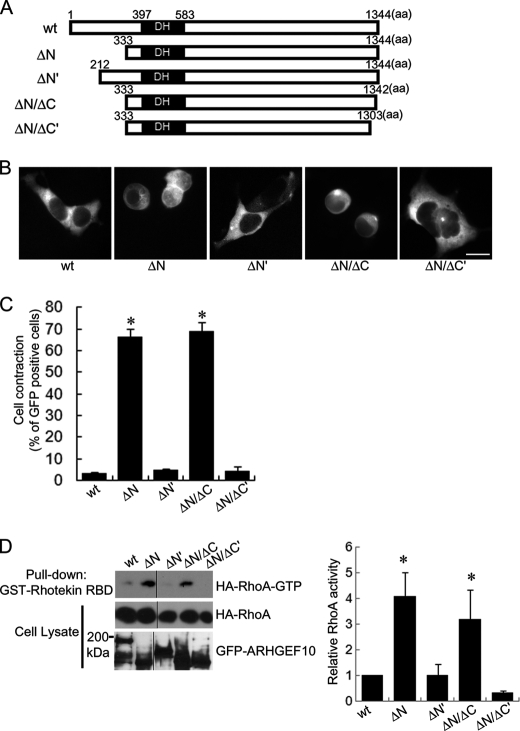
FIGURE 3.
Cell contraction and activation of RhoA by various deletion mutants of ARHGEF10 in HEK293T cells. A, domain structures of ARHGEF10. Full-length ARHGEF10 (wt), N-terminal truncation mutants lacking N-terminal 332 and 211 amino acids of ARHGEF10 (ΔN and ΔN′, respectively), and C-terminal truncation mutants lacking C-terminal 2 and 41 amino acids of ARHGEF10 ΔN (ΔN/ΔC and ΔN/ΔC′, respectively) are shown. B, HEK293T cells transiently transfected with the plasmid encoding GFP-ARHGEF10 wt, ΔN, ΔN′, ΔN/ΔC, or ΔN/ΔC′. After 48 h, fluorescence images of living cells observed. Scale bar, 10 μm. C, quantitative analyses of cell contraction of HEK293T cells. HEK293T cells were transiently transfected with the plasmid encoding GFP-ARHGEF10 wt, ΔN, ΔN′, ΔN/ΔC, or ΔN/ΔC′. The proportion of cell contraction was scored as a percentage of the rounded cells of GFP-positive cells. Cells floating in the culture medium were excluded from counting. The data represent the mean ± S.E. (error bars) from three independent experiments. *, p < 0.05 compared with wt. For each experiment, >100 cells were counted. D, HEK293T cells transiently co-transfected with the plasmids encoding GFP-ARHGEF10 wt, ΔN, ΔN′, ΔN/ΔC, or ΔN/ΔC′, and HA-RhoA. After 48 h, cells were lysed with lysis buffer. The supernatants were mixed with GST-Rhotekin-RBD, and an active form of RhoA was pulled down. The amounts of active form of RhoA were determined by immunoblotting with anti-HA antibody. Expressions of HA-RhoA and GFP-ARHGEF10 in cell lysates were also detected by immunoblotting with anti-HA and anti-GFP antibodies, respectively. The representative image of three independent experiments is shown. Relative RhoA activity is indicated by the amounts of RhoA-GTP normalized to total amounts of RhoA in cell lysates, and values are expressed as -fold value of cells transfected with wt. The data are the mean ± S.E. from three independent experiments. *, p < 0.05 compared with wt.