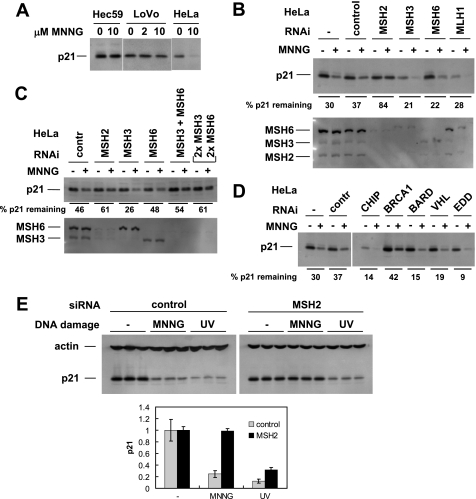
FIGURE 3.
MSH2 is required for p21 degradation in MNNG-treated cells. A, cell lines with the absence (Hec59, LoVo) or presence (HeLa) of MSH2 were tested for p21 degradation in response to MNNG treatment. Lack of MSH2 expression correlated with failure to degrade p21. B, in HeLa cells, MMR proteins were individually down-regulated by RNAi followed by MNNG treatment and Western blotting for p21. Only MSH2 knockdown consistently interfered with p21 degradation. C, in HeLa cells, MSH3 and MSH6 were targeted by RNAi individually and in combination. Only cells in which both were knocked down exhibited reduced degradation of p21 in response to MNNG. For the last pair of lanes, twice the amount of both MSH3 and MSH6 siRNAs was used (67 nm final concentration of siRNA on cells). contr, control. D, additional proteins known to be involved in protein degradation were tested in HeLa cells by RNAi for a role in the pathway that leads to p21 degradation in MNNG-treated cells. BRCA1 knockdown had a moderate but reproducible effect on p21 degradation. E, HeLa cells (triplicate samples) were transfected with either control or MSH2 siRNA. Three days later, the cells were treated with either MNNG or UV, and p21 degradation was assayed. Knockdown of MSH2 protected p21 from MNNG but not UV. Bottom panel, quantitation of p21 normalized to β-actin. Error bars in the bottom panel indicate S.D.