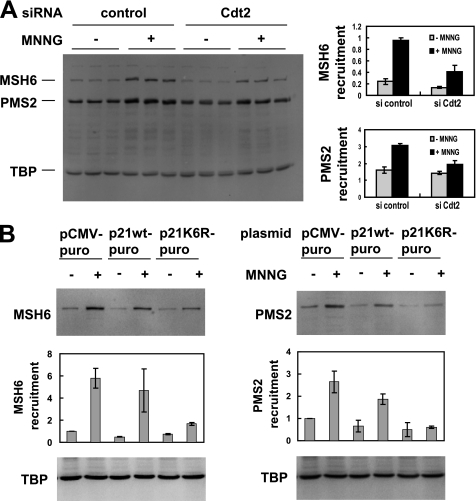
FIGURE 5.
Degradation of p21 is required for recruitment of MMR proteins in response to MNNG. A, Cdt2 was knocked down in HeLa cells using RNAi followed by MNNG treatment. Redistribution of MSH6 and PMS2 to DNA was assessed by extracting the cells with a Triton buffer. The remaining, non-extractable (mainly nuclear) proteins were subjected to SDS-PAGE and Western blotting. Cdt2 knockdown resulted in reduced nuclear recruitment of MSH6 and PMS2 in response to MNNG. Triplicate samples (independent wells in a 24-well plate) were quantitated (right panel), and average and S.D. are shown. TATA box-binding protein (TBP) was used as nuclear loading control and normalizer for the quantitation. B, HeLa cells were transiently transfected with either wild-type p21 or non-degradable p21K6R, and transfected cells were selected with puromycin. After MNNG treatment, redistribution of DNA repair proteins was assayed as in A. Expression of non-degradable p21 reduced recruitment of MSH6 (left panel) and PMS2 (right panel) to a Triton non-extractable (nuclear) fraction. Two separate experiments were performed that produced similar results; Western blots are from the first experiment, and quantitations show average and S.D. from both experiments.