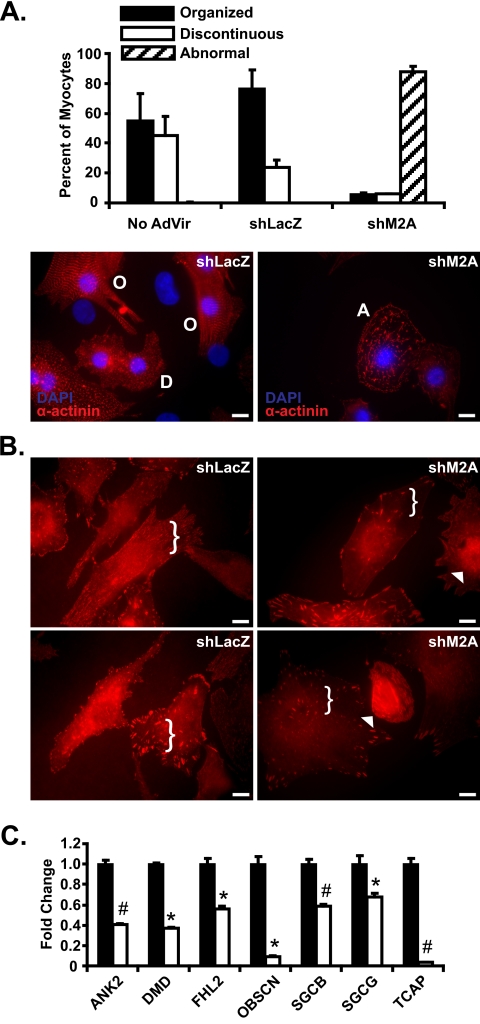
FIGURE 2.
RNA interference-mediated knockdown of Mef2A in neonatal cardiomyocytes results in structural and gene expression abnormalities. A, adenoviruses harboring either Mef2A short hairpin RNA (shM2A) or lacZ shRNA (shLacZ) were transduced into NRVM cultures at a multiplicity of infection of 20 and analyzed at 72 h post transduction. Left panel, immunofluorescence analysis with α-actinin antibodies in shLacZ transduced NRVMs revealed cells with either a highly organized (O) or discontinuous (D) α-actinin staining pattern. Right panel, shM2A cultures had a dramatic loss of the characteristic organized (O) and discontinuous (D) α-actinin stain with an increase in abnormal cells with fibrous, linear α-actinin stain (A). B, immunofluorescence analysis reveals mislocalization of vinculin in Mef2A knockdown cardiomyocytes. A reduction in the amount of punctate immunoreactivity is observed throughout the cell (brackets) and accompanied by an increase in perinuclear vinculin staining (arrowheads). C, costamere transcript analysis in shM2A NRVMs. qRT-PCR reveals a significant down-regulation in seven of the 12 costamere target genes. Total RNA was collected from six individual experiments and was performed in triplicate. *, p ≤ 0.05; #, p ≤ 0.008 versus shLacZ transduced cultures. Images for immunofluorescence experiments are representative of multiple experiments. Five hundred myocytes were counted for each transduced group (n = 3). Scale bars, 10 μm.