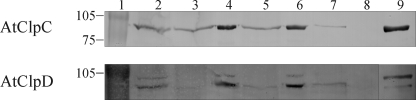
FIGURE 8.
Association of HSP100 proteins with import complexes containing TP-GST. TP-GST was incubated with intact chloroplasts in the presence of 100 μm ATP for 10 min at room temperature. After lysis, chloroplastic membranes were separated from soluble material by ultracentrifugation and solubilized in buffer containing DDM. Glutathione-agarose beads were added to capture TP-GST and associated proteins from the mixture. Samples corresponding to 50 μl of chloroplasts were analyzed by SDS-PAGE followed by immunoblotting using antibodies against AtClpC proteins (upper blot) or AtClpD (lower blot). Lane 1: molecular weight standards, lane 2: isolated chloroplasts, lanes 3 and 4: membrane and supernatant fractions from lysed chloroplasts, lanes 5 and 6: membrane and supernatant fractions from lysed chloroplasts with bound TP-GST, lane 7: import complexes containing bound TP-GST captured with glutathione-agarose resin, lane 8: as lane 7 but using GST instead of TP-GST, lane 9: purified recombinant protein. Representative blots from three different experiments are shown.