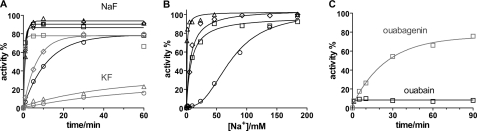
FIGURE 10.
Time course of reactivation of fluoride-inhibited Na,K-ATPase. A, time course of reactivation of Na,K-ATPase at 23 °C pH 7.5 by incubation in 150 mm NaCl prior to measurement of hydrolytic activity. The enzyme was treated with 5 mm NaF (black curves), or KF (gray curves) and either 5 mm MgCl2 (○), 5 μm BeSO4 (□), 200 μm AlCl3 (♢), 200 μm AlCl3 plus 1 mm ADP (△). The curves are monoexponential fits to the data. The observed rate constants, kobs are given in Table 2. The black symbols are with 5 mm NaF, and the gray symbols are with 5 mm KF as the fluoride donor. B, activity after 60 min reactivation as a function of Na+ concentration in the reactivation medium. Enzyme was treated with metal fluorides as indicated in panel A. The curves are fits using a one site binding model (hyperbola), or a sigmoid dose-response model. The fitted K0.5 values are given in Table 2. C, reactivation of BeF-treated enzyme with bound ouabain (10 μm, black curves) or ouabagenin (100 μm, gray curves). The CTS were included for 50 min in the metal fluoride incubation media before reactivation by 150 mm NaCl and activity test. The kobs for the reactivation in the presence of OG is 0.67·10−3 ± 0.04·10−3 s−1.