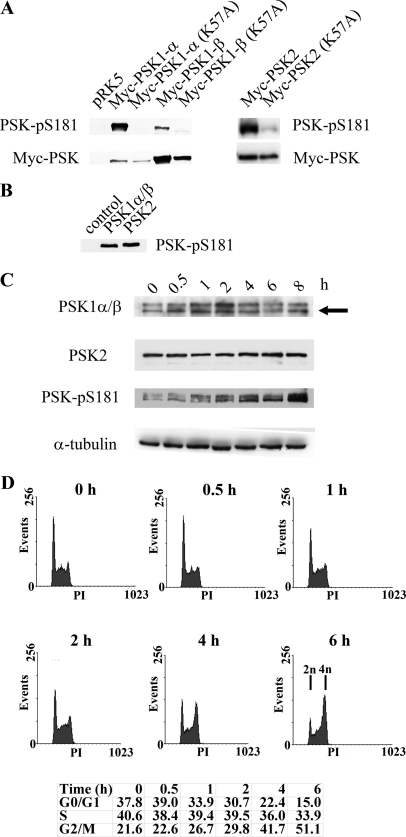
FIGURE 1.
A, PSK-Ser(P)-181 antibody detects catalytically active and phosphorylated PSK1-α, PSK1-β, and PSK2. Growing H1299 cells were transfected with pRK5-Myc vector, pRK5-Myc-PSK1-α, pRK5-Myc-PSK1-α (K57A), pRK5-Myc-PSK1-β, pRK5-Myc-PSK1-β (K57A), pRK5-Myc-PSK2, or pRK5-Myc-PSK2 (K57A). After 24 h, cell lysates were immunoblotted with anti-PSK-Ser(P)-181 antibody (upper panel) or anti-Myc antibody (lower panel), and proteins were detected by enhanced chemiluminescence. B, recombinant PSK1-α/β or PSK2 (amino acids 1–314, 30 ng) expressed and purified from Sf9 insect cells or controls without recombinant protein were subjected to in vitro kinase assays as described previously (9) and immunoblotted with the PSK-Ser(P)-181 antibody. C, nocodazole stimulates PSK activity and phosphorylation. Growing HeLa cells were treated with nocodazole (500 nm) for the times shown and then lysed and immunoblotted with antibodies to detect PSK1-α/β, PSK2, PSK-Ser(P)-181, or α-tubulin. PSK1-α/β proteins have similar mobilities, and the arrowed lower band represents both proteins (as confirmed using siRNA knockdown elsewhere in the manuscript) (“Results” and Fig. 7C). D, at the same times, some cells were fixed in 70% ethanol and stained with propidium iodide to determine cell cycle profiles using FACS and the percentages of cells in G0/G1, S, and G2/M are shown.