Abstract
Models of microgravity are linked to excessive constitutive nitric oxide (NO) synthase (NOS), splanchnic vasodilation, and orthostatic intolerance. Normal-flow postural tachycardia syndrome (POTS) is a form of chronic orthostatic intolerance associated with splanchnic hyperemia. To test the hypothesis that there is excessive constitutive NOS in POTS, we determined whether cutaneous microvascular neuronal NO and endothelial NO are increased. We performed two sets of experiments in POTS and control subjects aged 21.4 ± 2 yr. We used laser-Doppler flowmetry to measure the cutaneous response to local heating as an indicator of bioavailable neuronal NO. To test for bioavailable endothelial NO, we infused intradermal acetylcholine through intradermal microdialysis catheters and used the selective neuronal NOS inhibitor l-Nω-nitroarginine-2,4-l-diamino-butyric amide (Nω, 10 mM), the selective inducible NOS inhibitor aminoguanidine (10 mM), the nonspecific NOS inhibitor nitro-l-arginine (NLA, 10 mM), or Ringer solution. The acetylcholine dose response and the NO-dependent plateau of the local heating response were increased in POTS compared with those in control subjects. The local heating plateau was significantly higher, 98 ± 1%maximum cutaneous vascular conductance (%CVCmax) in POTS compared with 88 ± 2%CVCmax in control subjects but decreased to the same level with Nω (46 ± 5%CVCmax in POTS compared with 49 ± 4%CVCmax in control) or with NLA (45 ± 3%CVCmax in POTS compared with 47 ± 4%CVCmax in control). Only NLA blunted the acetylcholine dose response, indicating that NO produced by endothelial NOS was released by acetylcholine. Aminoguanidine was without effect. This is consistent with increased endothelial and neuronal NOS activity in normal-flow POTS.
Keywords: nitric oxide synthase isoforms, laser-Doppler flowmetry, acetylcholine, cutaneous blood flow
orthostatic intolerance is defined by symptoms and signs relieved by recumbency. Symptoms include dizziness, fatigue, exercise intolerance, headache, memory problems, palpitations, nausea, blurred vision, pallor, and abnormal sweating while upright, which improve with recumbence and which have no other medical explanation. Postural tachycardia syndrome (POTS) is the predominant form of chronic orthostatic intolerance (17, 28, 38, 52, 53) and is defined by symptoms of orthostatic intolerance associated with the physical sign of excessive upright tachycardia (38). We previously described three subsets of POTS patients.
Low-flow POTS is characterized by pallor, absolute hypovolemia, reduced stroke volume, blunted orthostatic vascular responses, increased plasma angiotensin II, and decreased bioavailability of cutaneous nitric oxide (NO) of neuronal NO synthase (nNOS) origin (46) related to increased plasma angiotensin II (44) consequent to angiotensin-converting enzyme 2 deficiency (50).
High-flow POTS is characterized by normovolemia, peripheral vasodilation, and increased peripheral blood flow, cardiac output, and microvascular filtration accounting for postural tachycardia (42, 43). Evidence indicated a mechanism of defective adrenergic-mediated vasoconstriction, possibly associated with a postviral peripheral neuropathy (16, 27).
In regard to normal-flow POTS, less well characterized are, perhaps, the majority of our recent POTS patients who have normal peripheral blood flow, heart rate, and vascular resistance when supine. Upright, splanchnic vasodilation causes splanchnic hyperemia, resulting in a redistributive reduction in central blood volume and reflex tachycardia because of excessive splanchnic pooling (45, 49).
Interestingly, splanchnic hyperemia also occurs in real and simulated microgravity and is often associated with orthostatic intolerance, adrenergic hyporeactivity (2, 26, 32).
We therefore hypothesized that microvascular NO production would be upregulated in normal-flow POTS subjects with splanchnic hyperemia. To test this hypothesis, we examined the plateau phase of the cutaneous response to local heat that is largely NO dependent (19, 27) and acetylcholine-induced increase in cutaneous blood flow that is due, in part, to endothelial NO (46). We used different doses of acetylcholine alone and then combined with NO synthase (NOS) inhibitors nitro-l-arginine (NLA), l-Nω-nitroarginine-2,4-l-diamino-butyric amide (Nω), and aminoguanidine (AG) to elicit changes in skin blood flow responses thought to represent local signaling by NO.
METHODS
To test this hypothesis, we used skin as a surrogate microvasculature (14) and stimulated the production of NO in two ways: 1) via the local cutaneous heating response reported by Kellogg et al. (21) and Minson et al. (29), which reaches a plateau that is highly sensitive to NOS inhibition; and 2) using the acetylcholine dose response, which is partly dependent on receptor-mediated endothelial NO production.
In both cases we tested our hypothesis by employing a highly selective, isoform-specific nNOS inhibitor Nω, a highly specific inducible NOS (iNOS) inhibitor AG, and a nonisoform-specific NOS inhibitor NLA to examine individual roles for nNOS, endothelial NOS (eNOS), and iNOS in the regulation of microvascular function in normal-flow POTS patients.
Subjects
Enrolled POTS patients were referred to the Hypotension Center for investigation of signs and symptoms of chronic orthostatic intolerance lasting at least 3 mo. Orthostatic intolerance was defined by the presence of dizziness, fatigue, exercise intolerance, headache, memory problems, palpitations, nausea, blurred vision, pallor, and abnormal sweating while upright, relieved by recumbence. The diagnosis of POTS was made in these patients during a screening upright tilt table test to 70° for a maximum of 10 min. POTS was diagnosed by symptoms of orthostatic intolerance during tilt associated with an increase in sinus heart rate of >30 beats/min or to a rate of >120 beats/min during the first 10 min of tilt (28, 36). During the same visit, POTS patients were partitioned on the basis of supine calf blood flow measured by venous occlusion strain-gauge plethymography (12) into either normal-flow, high-flow, or low-flow POTS as we have previously defined (48). In the current study, we only enrolled subjects with normal-flow POTS. Normal-flow POTS subjects are defined as those who had a minimum calf blood flow of 1.2 ml·100 ml tissue−1·min−1 and a maximum calf blood flow of 4.0 ml/100 ml tissue−1·min−1. This is based on the range of calf blood flow that we have measured in healthy volunteers from determinations in over 150 healthy subjects. Splanchnic hyperemia was confirmed in these patients (45, 49).
Using these criteria, we recruited 14 normal-flow POTS patients (10 women, 4 men; all Caucasian; age, 15.5–24.1 yr; and median age, 21.3 yr). Twelve healthy Caucasian volunteers subjects were also recruited (8 women, 4 men; all Caucasian; age, 17.0–25.7 yr; and median age, 21.5 yr) and were studied after a screening upright tilt at 70° demonstrated normal orthostatic response. Volunteer subjects served as a control group and were recruited from among adolescents and young adults referred for innocent heart murmur. Subjects with a history of syncope or orthostatic intolerance were specifically excluded from enrollment in this study.
All subjects were free from systemic, cutaneous, and cardiovascular diseases. Subjects refrained from alcohol and caffeinated beverages for 24 h before study and were not taking any medications. There were no smokers or competitive athletes. Informed consent was obtained from all participants, and the Committee for the Protection of Human Subjects (Institutional Review Board) of New York Medical College approved all protocols. Female subjects were enrolled without regard to the phase of their menstrual cycle except that none were menstruating during testing procedures.
Protocols
Two sets of experiments were performed in which changes in cutaneous blood flow were measured.
The first series of experiments investigated the cutaneous response to local heating in POTS patients compared with healthy volunteers in the presence and absence of NOS inhibition. We employed the highly selective nNOS (NOS-1) inhibitor Nω (10 mM), the highly selective iNOS (NOS-2) inhibitor AG (10 mM), and the nonselective NOS inhibitor NLA (10 mM). There is no highly selective eNOS (NOS-2) inhibitor, and eNOS inhibition was assessed by inference (e.g., suppression by NLA but not by Nω or AG) and by exploiting eNOS-specific stimulation using acetylcholine.
Past experiments have demonstrated that Nω decreased cutaneous vascular conductance (CVC) during local heating by an amount equivalent to the largest reduction produced by NLA (46). We have therefore used the local heating response as a bioassay for nNOS and do so in the current experimental design.
The second series of experiments investigated the response to increasing doses of acetylcholine, a receptor-mediated eNOS agonist. This was measured in the presence and absence of NOS inhibition.
Experiment 1: the effect of NOS inhibitors on local heat-mediated vasodilation.
We compared the effects of the isoform-selective nNOS inhibitor Nω to a maximally plateau-suppressing dose of NLA, a nonselective NOS inhibitor and to AG, a selective iNOS inhibitor. Nω is a highly specific nNOS inhibitor that does not bind the iNOS isoform and has ∼1,500-fold selectivity for nNOS over eNOS (15). AG is a highly specific iNOS inhibitor that has ∼100-fold selectivity for iNOS over nNOS or eNOS (57). All NOS inhibitors were dissolved in Ringer solution and, each having a low molecular weight, were freely permeable through the microdialysis dialysis catheters (see below).
Testing was conducted in a temperature-controlled room (∼25°C) at least 4 h after a light breakfast. Experiments began after a 30-min acclimatization period, and all experiments were performed while the subject was supine and resting comfortably. We used laser-Doppler flowmeters (Perimed, Stockholm, Sweden) with integrating laser-Doppler flow probes (Probe 413, Perimed) placed on the lateral aspect of the right calf to measure cutaneous blood flow (19). The laser-Doppler flow (LDF) probes were surrounded by a heating collar that enabled localized heating of the area under the flow probe. Measurements were made in the leg because prior experiments from our laboratory consistently indicate significant findings in the lower limb in POTS (16, 48). Blood pressure was monitored by Finometer (TNO, Amsterdam, The Netherlands), calibrated by automated oscillometry. Heart rate was monitored by continuous electrocardiogram. Continuous LDF data were collected at a sampling rate of 200 Hz. During experiments, data were multiplexed and interfaced to a personal computer through an analog-to-digital converter (DI-720, DATAQ industries, Milwaukee, WI), using custom software that generated binary files for all measurements while simultaneously displaying collected data on a computer screen.
LDF measurements were made on the right calf while supine with the leg at the level of the heart. Subjects were instrumented with four microdialysis catheters placed at least 6 cm apart, inserted in the dermal space of the lateral aspect of the calf after gentle hair removal. Each probe (MD-2000 Linear Microdialysis Probes, Bioanalytical Systems, West Lafayette, IN) has a 10-mm microdialysis membrane section that is placed in the intradermal space using a 25-gauge needle as an introducer. Catheters were randomly designated 1–4.
Following placement, all catheters were initially perfused with Ringer solution at 2 μl/min. An integrating laser-Doppler flow probe was placed directly over each microdialysis catheter to measure cutaneous LDF. There is a hyperemia following catheter insertion. LDF was recorded until values were similar to those measured over the same area before catheter insertion. The return of LDF to preinsertion values usually occurred by 60–90 min. When necessary, longer times were allowed until preinsertion LDF was achieved.
Once baseline LDF values were reached, all subjects received perfusate containing Ringer solution through catheter 1 (control), 10 mM NLA through catheter 2, 10 mM Nω through catheter 3, and 10 mM AG through catheter 4; each perfused at 2 μl/min for 45 min during this drug run-in phase. When run-in was complete, the area under each laser was heated at 1°C/10 s to 42°C for at least 40 min until a plateau was reached. Perfusion with Ringer solution or NOS inhibitors continued throughout the heating. At the end of the experiments, we maintained heating and perfused all catheters with 28 mM sodium nitroprusside to obtain maximum vasodilation and to compute the maximum CVC (CVCmax). CVC was calculated as the ratio of LDF to mean arterial blood pressure. CVCmax was defined as CVC during sodium nitroprusside infusion. Experimental results were thereafter expressed as %CVCmax(100·CVC/CVCmax).
Doses of 10 mM of NLA and 10 mM of Nω were chosen because these were the smallest concentrations of drugs that gave maximum suppression of the local heating plateau (46). Nω has a selectivity (Ki ratios) for nNOS/eNOS of 1,538 (15). At the dose used, and assuming similar microdialysis membrane permeability and interstitial diffusion, this is equivalent to 0.25% of the binding to eNOS and a fourfold greater binding to nNOS compared with equimolar NLA. The dose of AG used (10 mM) was based on pilot experiments (not shown) that determined the smallest dose required for a maximal effect. This was equimolar to the other NOS inhibitors used in the present study.
Experiment 2: the effect of NOS inhibitors on the acetylcholine-mediated vasodilation.
On another day, experiments were performed in the same subjects of experiment 1. Experiments were not conducted as classical “dose response” and “inhibition” studies since the derivation of pharmacological characteristics was not our intent. Rather, different doses of agonist (acetylcholine) alone and then combined with NOS inhibitors (NLA, Nω, and AG) were used to elicit changes in skin blood flow responses thought to represent local signaling by NO. We anticipated that NO would not completely suppress the acetylcholine response because there are effects of other local vasoactive agents, such as prostaglandins and endothelium-derived hyperpolarizing factor, and the influence of local axon reflexes.
Subjects were instrumented with four microdialysis catheters and integrative laser-Doppler probes as in experiment 1. Following recovery from catheter insertion, baseline LDF values were obtained. Subjects then received perfusate containing Ringer solution through catheter 1 (control), 10 mM NLA through catheter 2, 10 mM Nω through catheter 3, and 10 mM of AG through catheter 4, each perfused at 2 μl/min for 45 min during a drug run-in phase. When run-in was complete, subjects received perfusate containing acetylcholine, dissolved in Ringer solution containing identical NOS inhibitors (or Ringer alone) as received during run-in. Acetylcholine was perfused in ascending doses (0.01, 0.10, 1.0, 10, and 100 mM) in combination with NOS inhibitors through each catheter at 2 μl/min. The range of concentration of acetylcholine used (0.01–100 mM) was based on previous determinations in human skin using the delivery of this agonist through microdialysis catheters (24, 41). LDF monitoring continued and each dose was administered for 20 min during which steady-state values of LDF were achieved. For purposes of analysis, only the last 5 min of data were averaged at each acetylcholine dose.
At the end of experiments, all catheters were perfused with 28 mM sodium nitroprusside to obtain CVCmax.
Statistics
We used two-way analysis of variance (2 × 4) to compare the plateau phases of the local heating response treatment of POTS with those in control subjects receiving Nω, NLA, AG, or Ringer solution in experiment 1. We used two-way analysis of variance with repeated measures to compare dose-response curves of acetylcholine alone (acetylcholine + Ringer), acetylcholine + NLA, acetylcholine + Nω, and acetylcholine + AG for POTS and control subjects. The results were calculated using SPSS (Statistical Package for the Social Sciences) software version 14.0. Apart from representative figures, text, graphic, and Table 1, results are reported as means ± SE. Significance requires P < 0.05.
Table 1.
Dimensions and supine hemodynamics
| Control | POTS | |
|---|---|---|
| n | 12 | 14 |
| Age, yr | 22 ± 1.5 | 22 ± 1.8 |
| Weight, kg | 70 ± 3 | 68 ± 4 |
| Height, cm | 171 ± 2 | 169 ± 3 |
| Body surface area, m2 | 1.80±.04 | 1.76±.05 |
| Supine heart rate, beats/min | 68 ± 2 | 76 ± 4 |
| Supine systolic blood pressure, mmHg | 120 ± 3 | 124 ± 3 |
| Diastolic systolic blood pressure, mmHg | 67 ± 2 | 70 ± 3 |
| Pulse pressure, mmHg | 55 ± 2 | 54 ± 3 |
| Venous occlusion calf blood flow, ml · 100 ml−1 · min−1 | 2.5 ± 0.2 | 2.3 ± 0.2 |
| Calf arterial resistance, ml · 100 ml−1 · min−1 · mmHg−1 | 35 ± 4 | 38 ± 5 |
| Maximum laser-Doppler flow with sodium nitroprusside, pfu | 183 ± 10 | 167 ± 9 |
| Baseline laser-Doppler flow, pfu | 21 ± 5 | 17 ± 3 |
| Baseline, %CVCmax | 12 ± 1.1 | 11 ± 1.3 |
Values are means ± SE; n, number of subjects. POTS, postural tachycardia syndrome; %CVCmax, percent maximum cutaneous vascular conductance; pfu, perfusion units.
RESULTS
As shown in Table 1, POTS and control subjects had similar height, weight, supine heart rate, systolic blood pressure, diastolic blood pressure, pulse pressure, calf blood flow, and calf arterial resistance. The baseline LDF, the maximum LDF elicited with 28 mM sodium nitroprusside, and the calculated baseline %CVCmax were the same in POTS and control subjects. Microdialysis of drugs had no effect on systemic hemodynamics (heart rate, and arm and leg blood pressure) in any patient.
Experiment 1: The Effect of NOS Inhibitors on Local Heat-Mediated Vasodilation
The local heat response is increased in normal-flow POTS.
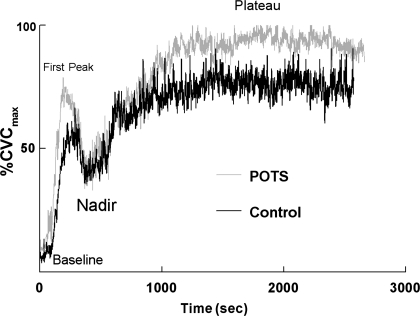
Figure 1 shows representative heating responses for a POTS patient and a control subject during local heating while perfused with Ringer solution (no NOS inhibition). The values for %CVCmax of the heat-induced plateau measured in the POTS subjects were nearly equal to 100%, whereas that of control subjects was significantly less.
Fig. 1.
Local heating response in a representative normal-flow postural tachycardia syndrome (POTS) patient (gray) and in a healthy volunteer control subject (black). Key points along the curve are marked. The heat response plateau, dependent on bioavailable nitric oxide (NO), is near maximum vasodilation in POTS patients, whereas the response in control subjects is significantly smaller. %CVCmax, percent maximum cutaneous vascular conductance.
NLA, Nω, and AG have no effect on baseline cutaneous blood flow.
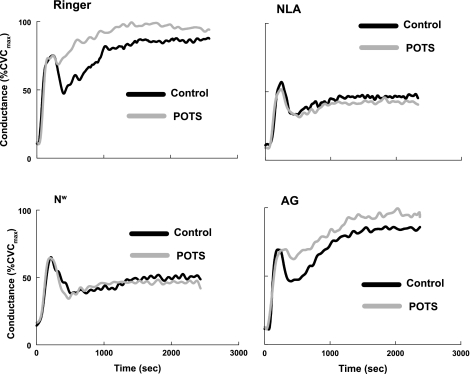
As shown in Fig. 2, and as previously reported for NLA (51) and Nω (46), the inhibition of NOS had no effect on the baseline cutaneous blood flow compared with Ringer alone when measured before the application of local heat. This was also found for AG, which had no effect on baseline flow, compared with that measured with Ringer solution alone. When each of these NOS inhibitors was tested, there was also no difference between baseline flows comparing control to POTS subjects.
Fig. 2.
Local heating responses averaged over all POTS patients (gray) and all control subjects (black). Results are shown when the NO synthase (NOS) inhibitors nitro-l-arginine (NLA), l-Nω-nitroarginine-2,4-l-diamino-butyric amide (Nω), or aminoguanidine (AG) dissolved in Ringer solution are given and when only Ringer solution is given. The NO-dependent plateau is larger than control in POTS (top, left). The plateau response is equally inhibited by the nonisoform-specific NOS inhibitor NLA (top, right) and by the neuronal NOS-specific NOS inhibitor Nω (bottom, left). AG (bottom, right) has no effect on the heating response, and results are similar to those using Ringer solution alone.
NLA and Nω are equipotent inhibitors of local heating in normal-flow POTS and control; AG has no effect on the local heating response.
Figure 2 shows heat responses averaged over all subjects for each NOS inhibitor in POTS and control subjects. On average, the plateau-phase conductance while receiving Ringer solution was 88 ± 2%CVCmax for control compared with 98 ± 1%CVCmax for normal-flow POTS (P < 0.01); the plateau phase conductance while receiving NLA was 47 ± 4%CVCmax for control compared with 45 ± 3%CVCmax for normal-flow POTS; the plateau phase conductance while receiving Nω was 49 ± 4%CVCmax for control compared with 46 ± 5%CVCmax for normal-flow POTS; and the plateau-phase conductance while receiving AG was 86 ± 2%CVCmax for control compared with 97 ± 2%CVCmax for normal-flow POTS (P < 0.025).
Conductance was significantly increased during local heating in normal-flow POTS, and this increase was unaffected by AG. NLA and Nω reduced the plateau conductance during local heating to a similar degree. During the perfusion of Ringer solution alone, the plateau conductance in normal-flow POTS patients was larger than the plateau conductance in control subjects. Consequently, perfusion with either NLA or Nω reduced the NO-sensitive plateau by a larger amount in normal-flow POTS compared with control subjects. Nω is as effective as NLA in blunting the hyperemia of local heating in both normal-flow POTS and control subjects. AG has no effect on any phase of the heat response.
Experiment 2. The Effect of NOS Inhibitors on the Acetylcholine-Mediated Vasodilation
The dose response to acetylcholine is increased in normal-flow POTS.
Figure 3 shows data averaged over all normal-flow POTS subjects and over all control subjects. Data showing the effect of acetylcholine dissolved in Ringer solution and free of NOS inhibitors are shown in Fig. 3, top, left. The ability of acetylcholine to increase cutaneous blood flow is significantly enhanced in POTS compared with control subjects (P < 0.001).
Fig. 3.
The dose response to logarithmic increases in perfused acetylcholine averaged over all POTS patients (gray) and all control subjects (black). Acetylcholine is perfused in combination with Ringer solution only or in combination with Ringer solution containing dissolved NOS inhibitors NLA, Nω, or AG. Results for acetylcholine plus Ringer solution are shown as solid lines and are present in each panel for comparison with the NOS inhibitor results shown as dashed lines. POTS increases the response to acetylcholine compared with control (top, left). Both POTS and control responses are similarly blunted by the addition of NLA but by neither Nω nor AG. *P < 0.05, significantly different from control; †P < 0.05, significantly different from baseline.
The dose response to acetylcholine is decreased by NLA but not Nω or AG in both normal-flow POTS and control subjects.
Figure 3 also demonstrates that NLA significantly (P < 0.0001) reduces the response to acetylcholine in both POTS and control subjects on the order of 50%. However, there was no significant difference in %CVCmax between control and POTS subjects when acetylcholine was administered in the presence of NLA. Consequently, perfusion with NLA reduced the response by a larger amount in POTS compared with control subjects.
There were no effects of selective nNOS and iNOS inhibitors on the acetylcholine dose response. There were large reductions of nonisoform selective NOS inhibition with NLA on the acetylcholine dose response.
DISCUSSION
Summary and Discussion of Findings
Our main findings are that cutaneous nNOS- and eNOS-mediated production of NO are both increased in normal-flow POTS patients compared with control subjects.
Experiment 1: nNOS activity is increased in normal-flow POTS.
The administration of a nonselective NOS inhibitor blunts the NO-dependent plateau of the local heating response. A selective nNOS inhibitor is equally effective in blunting this response at a dose that should exert a minimal effect on eNOS. AG has no effect on local heating, indicating a lack of influence of iNOS under these experimental conditions. These findings indicate that the local heating plateau can be used as a bioassay for nNOS activity. The local heating response is enhanced in normal-flow POTS compared with control subjects, reaching conductances close to CVCmax. This suggests that there is increased NO derived from nNOS in normal-flow POTS.
The dependence of the local heating response on nNOS is controversial. Kellogg et al. (22) have maintained that the local heating response is dependent on eNOS rather than nNOS. Those conclusions were based on observations made using 7-nitroindazole as a selective nNOS inhibitor and Nω-nitro-l-arginine as a selective eNOS inhibitor (22). However, 7-nitroindazole has no selectivity for nNOS in vitro (the IC50 values for inhibition of nNOS and eNOS are 0.71 and 0.78 μM, respectively) and exhibits modest selectivity in vivo. Nω-nitro-l-arginine inhibits nNOS with Ki values of 15 nM and eNOS with Ki value of 39 nM and cannot be regarded as eNOS selective.
Experiment 2: eNOS activity is increased in normal-flow POTS.
Our past work showed that acetylcholine increases cutaneous blood flow and that this increase is due, in part, to NO (46). Our current results are similar and thus support the association between increased blood flow and NO. While NLA does not completely eliminate the response to increasing doses of acetylcholine, it attenuates this increase by some 50% (Fig. 3).
The administration of a sufficient amount of the nonselective NOS inhibitor NLA blunts the response to acetylcholine. Neither the selective nNOS inhibitor Nω nor the selective iNOS inhibitor AG affects this acetylcholine-mediated response. These findings indicate that the blunting of the heat response by NLA is an indicator of local eNOS activity. The response to acetylcholine is enhanced in normal-flow POTS compared with control subjects. This suggests that there is increased NO of endothelial origin in normal-flow POTS. Other investigators have reported an increase in endothelial NO production during intradermal acetylcholine administration (5). In the current work, we found no effect of Nω at any dose of acetylcholine used.
Significance of Increased Bioavailable Constitutive NO
Systemic illnesses typically have cutaneous manifestations. In particular, prior cutaneous findings in other variants of POTS (50) have been substantiated by systemic measurements (30). The skin is therefore a useful surrogate of the microvasculature that is likely reflective of systemic disease (14) in POTS.
Both nNOS and eNOS are expressed constitutively (8) and require calcium-calmodulin for activation. nNOS was first discovered in the brain and therefore was initially associated with neurons, whereas eNOS was first discovered in endothelial cells. However, nNOS and eNOS have been found in diverse tissues. Thus, for example, nNOS has been found in keratinocytes (3); cardiac, skeletal muscle; and splanchnic, renal, and neurovascular tissue (6, 7, 18, 20), whereas eNOS has been found in every organ containing endothelium. nNOS has been shown to be important in the regulation of human basal forearm microvascular and coronary blood flow (39, 40).
Single nucleotide polymorphisms of eNOS have been explored in POTS and seem to be loosely related to disease (10), although studies did not subgroup patients on the basis of vascular physiology. Similar studies for nNOS single nucleotide polymorphisms and POTS have not yet been performed.
However, genetic differences are not necessary to alter the expression of constitutive NOS as evidenced in hepatic cirrhosis where both nNOS and eNOS isoforms are increased and sustain NO-mediated vasodilation and the hyperdynamic circulation (35). Changes in the expression of nNOS and eNOS have been reported following conditions of simulated microgravity and occur in both bed-rested humans and the rat hindlimb suspension models and are associated with splanchnic dilation (2, 56) and orthostatic intolerance (2, 54).
Increased NO can contribute to peripheral vasodilation through cGMP-mediated vasodilation (37), through its blunting of central sympathetic nerve activity (25) and through peripheral modulation of sympathetic neurotransmission. Modulated sympathetic neurotransmission may result from the inhibitory effects of NO on the release of norepinephrine (11), chemical deactivation of norepinephrine by NO reducing its activity (23), or both. Of particular interest are the effects of nNOS on the central sympathetic nervous system and on nitrergic nerves innervating mesenteric vasculature (13, 34). Nitrergic nerves contain nNOS and are distributed with cholinergic parasympathetic nerves. nNOS may even colocalize with acetylcholine and vasoactive intestinal peptide in the perivascular parasympathetic fibers (33) and modulate vascular tone and blood flow (31, 55).
An Interpretation for the Mechanistic Pathophysiology of Normal-Flow POTS Patients
Data from these and other experiments can tentatively provide an explanation of the pathophysiology of normal-flow POTS. Our previous data have shown an increase in splanchnic blood flow and splanchnic blood volume (45, 49) in normal-flow POTS, and the current data support an increase in neuronal and endothelial NO. Globally increased neuronal NO might act to reduce sympathoexcitation both supine and upright, but there is no clinical evidence for a reduction of sympathetic nerve activity in POTS (4). Therefore, the results are most consistent with the interference with the peripheral adrenergic vasoconstriction. This again is based on the assumption that the results obtained in skin are reflective of microvascular function elsewhere.
As we have previously reported, reduced vasoconstriction in normal-flow POTS seems limited to the splanchnic circulation while patients are in an upright position. How adrenergic hyporeactivity may be limited by posture to the regional vasculature remains to be verified in future systemic experiments.
Summary
We used laser-Doppler flowmetry to measure the cutaneous response to local heating as an indicator of bioavailable neuronal NO. The acetylcholine dose response and the NO-dependent plateau of the local heating response were increased in POTS compared with control subjects. The local heating plateau was significantly higher in POTS compared with control but decreased to the same level with Nω or with NLA. Only NLA blunted the acetylcholine dose response, indicating that NO produced by eNOS was released by acetylcholine. This suggests increased eNOS and nNOS activity in normal-flow POTS subjects.
Limitations
We studied the cutaneous circulation, which has unique autonomic control. Our recent work indicates that flow regulation abnormalities in POTS occur throughout the circulatory system (49) and that the flow abnormalities that occur in skin may be used as representation of systemic diseases (14).
We studied the calf cutaneous circulation. While flow abnormalities are widespread in POTS, whenever peripheral blood flows are studied, the most significant results occur in the lower extremities. It may be that dependence and gravitational exposure are important to the observed findings. There may be inherent differences in forearm versus calf cutaneous microvascular control that may explain the role of various NOS isoforms thought to be responsible for the mediation of the local heat response (22, 46).
Whereas Nω has well-documented in vitro selectivity for nNOS over eNOS (15), in vivo selectivity has not been well established. Thus there could be additional actions of this agent on choline transport, acetylcholinesterase, butyrocholinesterase, muscarinic, prostaglandin, or NO mechanisms other than its effects of NOSs.
We studied women without regard to menstrual cycle. The phase of the menstrual cycle can exert important effects on NO-dependent mechanisms (1). Recent evidence suggests a relationship between phases of the menstrual cycle and changes in POTS symptoms and signs during a 2-h stand because of altered response of the renal-angiotensin-aldosterone system that affects hemodynamics during orthostasis (9). Since our determinations are made while the subject is supine, it is unlikely that hormone-related differences play a major role in the response of skin to local heat; however, this issue remains a concern.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-087803 and RO1-HL-074873.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 235: 111–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arbeille PP, Besnard SS, Kerbeci PP, Mohty DM. Portal vein cross-sectional area and flow and orthostatic tolerance: a 90-day bed rest study. J Appl Physiol 99: 1853–1857, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Baudouin JE, Tachon P. Constitutive nitric oxide synthase is present in normal human keratinocytes. J Invest Dermatol 106: 428–431, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Bonyhay I, Freeman R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110: 3193–3198, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Boutsiouki P, Georgiou S, Clough GF. Recovery of nitric oxide from acetylcholine-mediated vasodilatation in human skin in vivo. Microcirculation 11: 249–259, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Danson EJ, Mankia KS, Golding S, Dawson T, Everatt L, Cai S, Channon KM, Paterson DJ. Impaired regulation of neuronal nitric oxide synthase and heart rate during exercise in mice lacking one nNOS allele. J Physiol 558: 963–974, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dawson D, Lygate CA, Zhang MH, Hulbert K, Neubauer S, Casadei B. nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation 112: 3729–3737, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Forstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J 12: 773–790, 1998 [PubMed] [Google Scholar]
- 9. Fu Q, VanGundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension 56: 82–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garland EM, Winker R, Williams SM, Jiang L, Stanton K, Byrne DW, Biaggioni I, Cascorbi I, Phillips JA, 3rd, Harris PA, Rudiger H, Robertson D. Endothelial NO synthase polymorphisms and postural tachycardia syndrome. Hypertension 46: 1103–1110, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Greenberg SS, Cantor E, Diecke FP, Peevy K, Tanaka TP. Cyclic GMP modulates release of norepinephrine from adrenergic nerves innervating canine arteries. Am J Hypertens 4: 173–176, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Greenfield AD, Whitney RJ, Whitney RJ. Methods for the investigation of peripheral blood flow. Br Med Bull 19: 101–109, 1963 [DOI] [PubMed] [Google Scholar]
- 13. Hatanaka Y, Hobara N, Honghua J, Akiyama S, Nawa H, Kobayashi Y, Takayama F, Gomita Y, Kawasaki H. Neuronal nitric-oxide synthase inhibition facilitates adrenergic neurotransmission in rat mesenteric resistance arteries. J Pharmacol Exp Ther 316: 490–497, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol 105: 370–372, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Huang H, Martasek P, Roman LJ, Silverman RB. Synthesis and evaluation of peptidomimetics as selective inhibitors and active site probes of nitric oxide synthases. J Med Chem 43: 2938–2945, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D. The neuropathic postural tachycardia syndrome. N Engl J Med 343: 1008–1014, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 96: 575–580, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Jansson L, Carlsson PO, Bodin B, Andersson A, Kallskog O. Neuronal nitric oxide synthase and splanchnic blood flow in anaesthetized rats. Acta Physiol Scand 183: 257–262, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol 56: 798–803, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol 97: 293–301, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Kellogg DL, Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol 107: 1438–1444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol 286: H296–H303, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Lee K, Mack GW. Role of nitric oxide in methacholine-induced sweating and vasodilation in human skin. J Appl Physiol 100: 1355–1360, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Liu JL, Murakami H, Zucker IH. Angiotensin II-nitric oxide interaction on sympathetic outflow in conscious rabbits. Circ Res 82: 496–502, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Lobachik VI, Chupushtanov SA, Pishchulina GI. Mechanisms of the decrease in human postural stability during prolonged head-down tilt. J Gravit Physiol 11: 139–140, 2004 [PubMed] [Google Scholar]
- 27. Low PA. Autonomic neuropathies. Curr Opin Neurol 15: 605–609, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS). Neurology 45: S19–S25, 1995 [PubMed] [Google Scholar]
- 29. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Mustafa HI, Garland EM, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm 8: 422–428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okamura T, Ayajiki K, Uchiyama M, Uehara M, Toda N. Neurogenic vasodilatation of canine isolated small labial arteries. J Pharmacol Exp Ther 288: 1031–1036, 1999 [PubMed] [Google Scholar]
- 32. Overton JM, Tipton CM. Effect of hindlimb suspension on cardiovascular responses to sympathomimetics and lower body negative pressure. J Appl Physiol 68: 355–362, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Parsons RL, Locknar SA, Young BA, Hoard JL, Hoover DB. Presence and co-localization of vasoactive intestinal polypeptide with neuronal nitric oxide synthase in cells and nerve fibers within guinea pig intrinsic cardiac ganglia and cardiac tissue. Cell Tissue Res 323: 197–209, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain Res 734: 109–115, 1996 [PubMed] [Google Scholar]
- 35. Rockey DC, Shah V. Nitric oxide biology and the liver: report of an AASLD research workshop. Hepatology 39: 250–257, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc 74: 1106–1110, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta 1178: 153–175, 1993 [DOI] [PubMed] [Google Scholar]
- 38. Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 43: 132–137, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation 119: 2656–2662, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation 117: 1991–1996, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol 93: 1947–1951, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation 105: 2274–2281, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Stewart JM. Microvascular filtration is increased in postural tachycardia syndrome. Circulation 107: 2816–2822, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond) 110: 255–263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 290: H665–H673, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293: H2161–H2167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 285: H2749–H2756, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 287: H1319–H1327, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1–7) production in postural tachycardia syndrome. Hypertension 53: 767–774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stewart JM, Taneja I, Glover J, Medow MS. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 294: H466–H473, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Streeten DH. Pathogenesis of hyperadrenergic orthostatic hypotension. Evidence of disordered venous innervation exclusively in the lower limbs. J Clin Invest 86: 1582–1588, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Streeten DH, Anderson GH, Jr, Richardson R, Thomas FD. Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling. J Lab Clin Med 111: 326–335, 1988 [PubMed] [Google Scholar]
- 54. Tarasova O, Borovik A, Tsvirkoun D, Lebedev V, Steeves J, Krassioukov A. Orthostatic response in rats after hindlimb unloading: effect of transcranial electrical stimulation. Aviat Space Environ Med 78: 1023–1028, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Toda N, Okamura T. The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev 55: 271–324, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Vaziri ND, Ding Y, Sangha DS, Purdy RE. Upregulation of NOS by simulated microgravity, potential cause of orthostatic intolerance. J Appl Physiol 89: 338–344, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Wolff DJ, Lubeskie A. Aminoguanidine is an isoform-selective, mechanism-based inactivator of nitric oxide synthase. Arch Biochem Biophys 316: 290–301, 1995 [DOI] [PubMed] [Google Scholar]