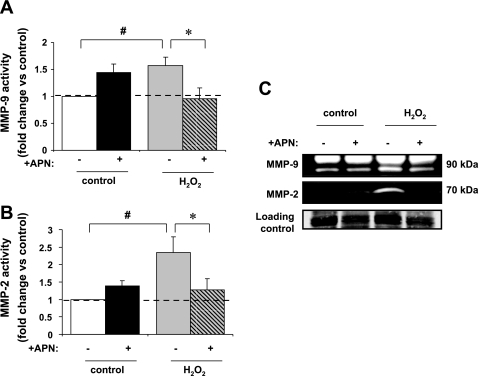
Fig. 2.
In-gel zymography assessment of matrix metalloproteinase (MMP)-9 and MMP-2 activity. A: MMP-9 gelatinase activity was localized to ∼90 kDa. MMP gelatinase activity in left ventricular (LV) myocyte-conditioned media was increased 57 ± 16% after treatment with H2O2 (#P < 0.01 vs. control). APN pretreatment completely inhibited the increase in MMP-9 activity in response to H2O2 (*P < 0.05 vs. H2O2 alone; n = 5). B: MMP-2 gelatinase activity was localized to ∼70 kDa. MMP gelatinase activity was increased by 1.4 ± 0.5-fold after treatment with H2O2 (#P < 0.01 vs. control). Increase in MMP-2 activity seen with H2O2 was significantly reduced by APN pretreatment (*P < 0.05 vs. H2O2 alone; n = 6). C: representative gelatin zymogram of MMP activity in conditioned media taken from untreated LV myocytes and those treated with 10 μM H2O2 and/or pretreated with APN (30 μg·ml−1·18 h−1; 105 total cells; n = 5–6). MMP gelatinolytic activity was observed between 100 and 50 kDa, which is consistent with MMP-2 and MMP-9.