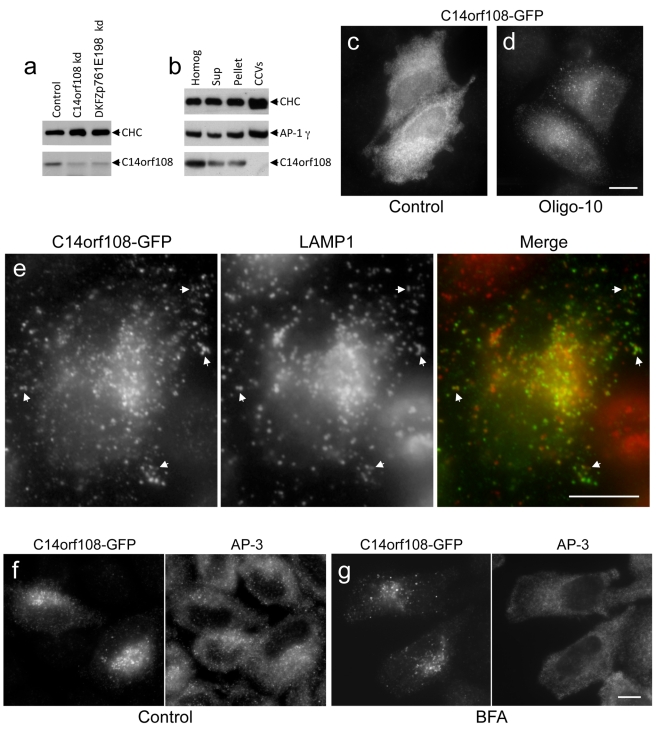
Figure 3. Characterisation of C14orf108 by Western blotting and immunofluorescence.
(a) A rabbit antiserum raised against a C14orf108-derived fusion protein recognises a band of the appropriate size on Western blots of HeLa cell homogenates, which is reduced in intensity when the cells are treated with SMARTpool siRNAs targeting the protein, and also when cells are treated with SMARTpool siRNAs targeting DKFZp761E198. For this and all subsequent siRNA experiments, control cells were treated with RISC-free control siRNA. (b) C14orf108 partitions between membranes and cytosol when cell homogenates are centrifuged at high speed, and does not appear to be associated with clathrin-coated vesicles (CCVs). (c) In cells transfected with GFP-tagged C14orf108, most of the construct is cytosolic. (d) If the cells are treated with Oligo-10, an siRNA that depletes endogenous C14orf108, the tagged construct has a punctate distribution in a limited number of cells. (e) Double labelling for tagged C14orf108 and LAMP1 in cells treated with Oligo-9. By moderating protein levels, treatment with Oligo-9 highlights the membrane association of the construct. There is substantial overlap between tagged C14orf108 and LAMP1. (f and g) Cells treated with Oligo-9 were double labelled for tagged C14orf108 and AP-3, either without (f) or with (g) a 5-min incubation in 20 µM brefeldin A (BFA). Unlike AP-3, C14orf108 is insensitive to BFA. The need to use anti-GFP, instead of relying on GFP fluorescence, indicates that AP-5 is not very abundant. Scale bars: 20 µm.