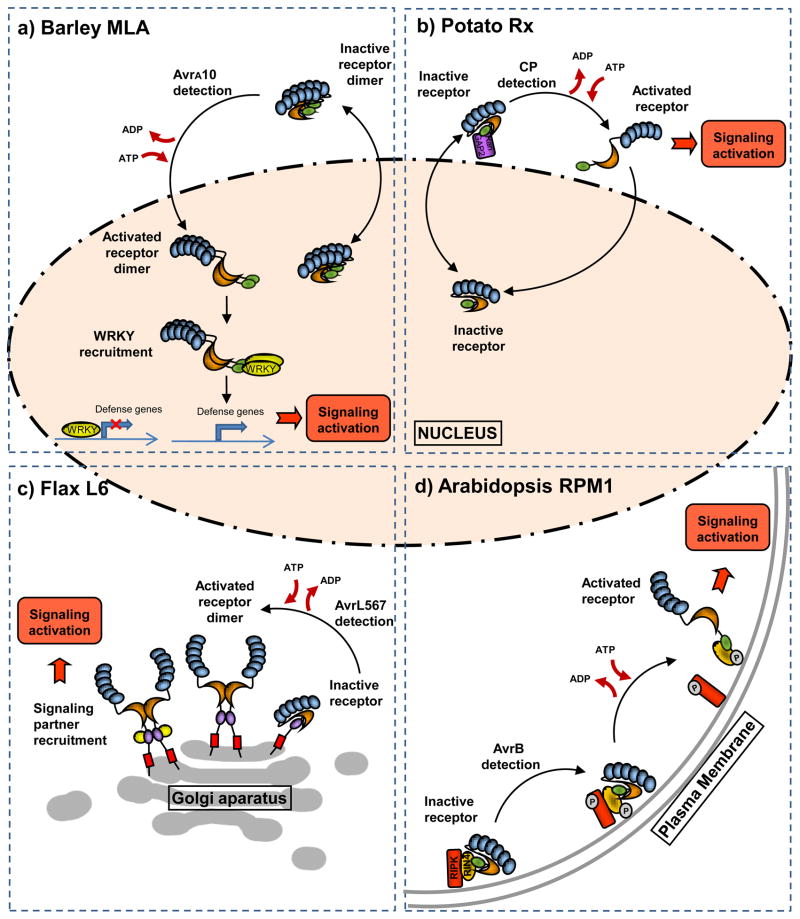
Figure 1. Different models for NB-LRR signaling activation.
Models for activation and signalling are presented for several R proteins to illustrate the potential variability in the mechanism and subcellular location of these events. The R protein N-terminal TIR and CC domains are represented by purple and green ovals respectively, the NB-ARC domain by an orange crescent and the Leucine Rich Repeats by a series of blue ovals.
a) In a resting state, Barley MLA navigates between the cytosol and the nucleus as an inactive homodimer interacting through the CC domain. The presence of its corresponding Barley mildew effector AvrA10 induces the accumulation of MLA in the nucleus, nucleotide exchange and conformational changes allowing the interaction of the CC domain with WRKY factors to derepress defense activation. b) The potato CC-NB-LRR Rx is also present in both the nucleus and cytosol. Its nucleocytoplasmic partitioning depends on the trafficking regulator RanGAP2 (purple), which acts as a cytoplasmic retention factor of Rx. The Potato Virus X Coat Protein (CP) is recognized in the cytosol and signaling is activated in this location. The Rx nuclear pool is required for correct regulation of resistance function. This protein is shown as a monomer as no direct evidence of its oligomerisation state is available. c) In the absence of pathogen, L6 is attached to the Golgi membrane through its N-terminal signal anchor (red rectangle). The protein is kept in an inactive state where the TIR domain dimerization interface is not exposed. Upon recognition of the flax rust effector AvrL567, nucleotide exchange and conformational change exposes the TIR domain for homodimerization and interaction with signaling proteins (yellow circles) to activate defense signalling. d) The Arabidopsis RPM1 protein is kept at the plasma membrane in a complex with the effector target RIN4 (yellow) and the protein kinase RIPK (red). The presence of the Pseudomonas effector AvrB induces RIPK and RIN4 phosphorylation. RIN4 modification leads to RPM1 activation and signaling at the plasma membrane. Again, in the absence of direct evidence otherwise, this protein is shown as a monomer..